Abstract
9-Methyl-3,5-dioxo-4-azatricyclo[5.2.2.02,6]undec-8-ene-1,8-diyl diacetate was synthesized from 2-methylcyclohexane-1,3-dione and 1H-pyrrole-2,5-dione. The title compound was characterized by 1H NMR, 13C NMR, elemental analysis and MS.
1. Introduction
Cyclic imides are extensively used as analgesic [1] and antinociceptive agents [2], or as reactants for polymer synthesis [3]. An imide nucleus can be also found in a structure of anxiolytic [4], antimicrobial [5], anticancer and anti-inflammatory substances [6,7]. They are also the objects of quantum chemical studies [8]. Several techniques to produce cyclic imides were described. Thus, unsubstituted cyclic anhydrides are successfully subjected to the reaction with ammonia, urea, formamide, lithium nitride or ammonium carbonate under mild conditions [9,10,11]. Substituted polycyclic imide rings are usually prepared in the Diels-Alder reaction [12,13]. Currently, catalyzed syntheses conducted under microwave irradiation have been described [14]. This work describes a conventional method for the synthesis of the substituted 4-azatricyclo-[5.2.2.02,6]undecene derivative.
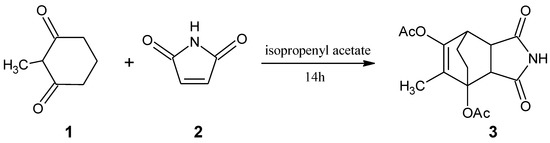
Scheme 1.
Synthesis of 9-methyl-3,5-dioxo-4-azatricyclo[5.2.2.02,6]undec-8-ene-1,8-diyl diacetate.
2. Experimental
2.1. General
All chemicals and solvents were purchased from Sigma-Aldrich (Vienna, Austria). Melting points were determined on an Electrothermal Digital Melting Point Apparatus (Essex, UK) and are uncorrected. The NMR spectra were recorded on a Bruker (Rheinstetten, Germany) spectrometer. The chemical shift values are expressed in ppm relative to TMS as an internal standard. Elemental analysis was recorded on a CHN model 2400 Perkin-Elmer (Hitachi, Tokyo, Japan). Mass spectra were performed on MARINER PE Biosystems instrument (Foster City, USA) with TOF detector. Methanol was used as a solvent. The spectra were performed in the positive ion mode with a declustering potential 140–300 V. TLC was carried out using silica gel 60 F254, layer thickness 0.25 mm (E. Merck, Darmstadt, Germany) and the results were visualized using UV lamp at 254 nm.
2.2. Synthesis of 9-methyl-3,5-dioxo-4-azatricyclo[5.2.2.02,6]undec-8-ene-1,8-diyl diacetate (3)
A mixture of 2-methylcyclohexane-1,3-dione (1) (5 g, 0.040 mol), 1H-pyrrole-2,5-dione (2) (4.62 g, 0.048 mol), and 4-methylbenzenesulfonic acid (0.05 g, 0.0003 mol) was dissolved in 15 mL of isopropenyl acetate and refluxed for 14 h. The solvent was evaporated. The crude product was crystallized from a hexane:ethyl acetate mixture (1:1 vol.) to afford a colourless solid.
Yield: 58%.
M.P. 161–162 °C.
1H NMR (400 MHz, DMSO-d6): δ = 11.18 (s, 1H, NH), 4.00 (d, J = 8.4 Hz, 1H, CH-C=O), 3.07 (dd, J1 = 2.8 Hz, J2 = 8.4 Hz, 1H, CH-C=O), 2.81 (d, J = 2.4 Hz, 1H, CH-C=C), 2.45 (m, 1H, CH2CH2), 2.14 (s, 3H, CH3-C=O), 2.09 (s, 3H, CH3-C=O), 1.73 (m, 2H, CH2CH2), 1.55 (s, 3H, CH3), 1.47 (dd, J1 = 4.4 Hz, J2 = 12.0 Hz, 1H, CH2CH2).
13C NMR (100 MHz, DMSO-d6): δ = 178.60 (C=O), 176.94 (C=O), 169.67 (C=O), 168.58 (C=O), 142.46 (C=C), 123.18 (C=C), 81.07 (C), 45.35 (CH), 44.41 (CH), 35.36 (CH), 27.64 (CH2), 23.18 (CH2), 21.87 (CH3), 21.16 (CH3), 9.45 (CH3).
HR ESI-MS: m/z [%]: 330.0954 [M + Na]+ 100.
Anal. Calcd. (found) for C15H17NO6 (307.30): C, 58.63 (58.68); H, 5.58 (5.62); N, 4.56 (4.58).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6References
- Borchhardt, D.M.; Andricopulo, A.D. CoMFA and CoMSIA 3D QSAR models for a series of cyclic imides with analgesic activity. Med. Chem. 2009, 5, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hao, G.F.; Tan, Y.; Xi, Z.; Huang, M.Z.; Yang, G.F. Bioactive conformation analysis of cyclic imides as protoporphyrinogen oxidase inhibitor by combining DFT calculations, QSAR and molecular dynamic simulations. Bioorg. Med. Chem. 2009, 17, 4935–4942. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Vittal, R.; Nien, P.C.; Liou, G.S.; Ho, K.C. A novel molecularly imprinted polymer thin film as biosensor for uric acid. Talanta 2010, 80, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Bojarski, A.J.; Kuran, B.; Kossakowski, J.; Kozioł, A.; Jagiełło-Wójtowicz, E.; Chodkowska, A. Synthesis and serotonin receptor activity of the arylpiperazine alkyl/propoxy derivatives of new azatricycloundecanes. Eur. J. Med. Chem. 2009, 44, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Struga, M.; Kossakowski, J.; Stefańska, J.; Zimniak, A.; Kozioł, A. Synthesis and antibacterial activity of bis-[2-hydroxy-3-(1,7,8,9,10-pentamethyl-3,5-dioxo-4-aza-tricyclo[5.2.1.02,6]dec-8-en-4-yloxy)-propyl]-dimethyl-ammonium chloride. Eur. J. Med. Chem. 2008, 43, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Yunesa, J.A.; Cardoso, A.A.; Yunes, R.A.; Corrêa, R.; de Campos-Buzzi, F.; Filho, V.C. Antiproliferative effects of a series of cyclic imides on primary endothelial cells and a leukemia cell line. Z. Naturforsch. C 2008, 63, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, S.M.; Rani, R.; Roy, P.; Agrawal, S.K.; Saxena, A.K. Microwave-assisted synthesis of N-substituted cyclic imides and their evaluation for anticancer and anti-inflammatory activities. Bioorg. Med. Chem. Lett. 2009, 19, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, L.; Yang, G.F.; Zhan, C.G. Quantitative structure-activity relationship for cyclic imide derivatives of protoporphyrinogen oxidase inhibitors: a study of quantum chemical descriptors from density functional theory. J. Chem. Inf. Comp. Sci. 2004, 44, 2099–2105. [Google Scholar] [CrossRef] [PubMed]
- Handley, G.J.; Nelson, E.R.; Somers, T.C. Compounds derived from β-substituted glutaric acids: glutarimides, glutaramic acids, 1,5-pentanediols. Aust. J. Chem. 1960, 13, 127–144. [Google Scholar] [CrossRef]
- Polonaski, T.; Milewska, M.J.; Gdaniec, M. Synthesis, structure and chiroptical spectra of the bicyclic α-diketones, imides and dithioimides related to santenone. Tetrahedron Asymmetry 2000, 11, 3113–3122. [Google Scholar] [CrossRef]
- Gordon, A.J.; Ehrenkaufer, R.L.E. Chemistry of imides. II. Cyclic imides and some unusual products from some diacid chlorides and lithium nitride. J. Org. Chem. 1971, 36, 44–45. [Google Scholar] [CrossRef]
- Ogbomo, S.M.; Burnell, D.J. cis-3,5-Cyclohexadiene-1,2-diol derivatives: facial selectivity in their Diels-Alder reactions with ethylenic, acetylenic and azo dienophiles. Org. Biomol. Chem. 2006, 4, 3838–3848. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.W.; Pool, B.R.; White, J.M. Structural studies on cycloadducts of furan, 2-methoxyfuran, and 5-trimethylsilylcyclopentadiene with maleic anhydride and N-methylmaleimide. J. Org. Chem. 2008, 73, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.; Hijji, Y. The synthesis of unsubstituted cyclic imides using hydroxylamine under microwave irradiation. Molecules 2008, 13, 157–169. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).