Abstract
The title compound, (2E)-3-(3,5-dimethyl-1-phenyl-1H-pyrazol-4-yl)-1-(2,5-dimethyl-3-furanyl)prop-2-en-1-one (3) was synthesized in high yield by an aldol condensation between 3-acetyl-2,5-dimethylfuran and 3,5-dimethyl-1-phenylpyrazole-4-carboxaldehyde in ethanolic NaOH at room temperature. Its structure was fully characterized by elemental analysis, IR, 1H NMR, 13C NMR and EI-MS spectral analysis.
Chalcones are also known as 1,3-diaryl-2-propen-1-ones, they belong to the flavonoid family. Chemically they consist of open-chain flavonoids in which the two aromatic rings are joined by a three-carbon α,β-unsaturated carbonyl system [1]. Chalcones have been reported to possess many useful properties, including anti-inflammatory [2], antimicrobial [3], antimitotic [4] antifungal [5], antioxidant [6] and anticancer activities [7]. Cyclization of chalcones such as pyrazoline can dramatically increase the biological activity such as antibacterial [8], antifungal [9], antiprotozoal [10], anti-inflammatory [11]. On the basis of these aspects, pyrazoline-containing chalcones should exhibit interesting biological activity. In this paper, we are reporting the synthesis of a novel pyrazoline-containing chalcone from 3-acetyl-2,5-dimethylfuran and 3,5-dimethyl-1-phenylpyrazole-4-carbox-aldehyde.
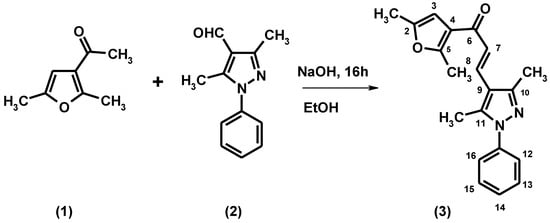
Figure 1.
Synthesis of the title compound.
A solution of 3-acetyl-2,5-dimethylfuran (0.33 mL, 0.0025 mol) and 3,5-dimethyl-1-phenyl-pyrazole-4-carboxaldehyde (0.50 g, 0.0025 mol) in an ethanolic solution of NaOH (6 g in 10 mL of ethanol) was stirred for 16 h at room temperature. The solution was poured into ice-cold water of pH~2 (pH adjusted by HCl). The solid was separated and dissolved in CH2Cl2, washed with a saturated solution of NaHCO3 and evaporated to dryness. The residue was recrystallized from methanol/chloroform to give a light-yellow solid.
Yield: 75%; m.p. 109–110 °C
ESI-MS m/z (rel. int.%): 321 (72) [M+1]+
IR (KBr) vmax cm-1: 3043 (C-Haromatic), 2926 (C-Haliphatic), 1636 (C=O), 1562 (C=C).
1H NMR (600 MHz, CDCl3) (δ/ppm): 7.79 (d, 1H, C7, C=CH, J = 15.6 Hz,), 6.93 (d, 1H, C8, CO=CH, J = 15.6 Hz), 7.27 (s, 1H, C3, CH, furan), 7.49–6.90 (m, 5H, Ar-H), 2.63 (s, C2, CH3), 2.51 (s, C5, CH3), 2.43 (s, C10, CH3), 2.22 (s, C11, CH3).
13CNMR (150 MHz, CDCl3) δ: 185.99, 157.43, 149.92, 149.21, 141.08, 138.92, 134.11, 129.24, 128.18, 125.17, 124.28, 122.67, 121.08, 115.25, 114.32, 105.24, 14.51, 14.29, 13.28, 11.60.
Anal. calc. for C20H20N2O2: C, 78.98, H, 6.29, N, 8.74; Found: C, 78.93, H, 6.21, N, 8.72.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The authors would like to thank the deanship of scientific research for the financial support of this work via Grant No. (3-045/430).
References
- Nowakowska, Z. A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med Chem. 2007, 42, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Avila, H.P.; Smania, E.F.A.; Monache, F.D.; Smania, A., Jr. Structure–activity relationship of antibacterial chalcones. Bioorg. Med. Chem. 2008, 16, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Batovska, D.; Parushev, S.; Stamboliyska, B.; Tsvetkova, I.; Ninova, M.; Najdenski, H. Examination of growth inhibitory properties of synthetic chalcones for which antibacterial activity was predicted. Eur. J. Med. Chem. 2009, 44, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Ducki, S.; Forrest, R.; Hadfield, J.A.; Kendall, A.; Lawrence, N.J.; McGown, A.T.; Rennison, D. Potent antimitotic and cell growth inhibitory properties of substituted chalcones. Bioorg. Med. Chem. Lett. 1998, 8, 1051–1056. [Google Scholar] [CrossRef]
- Lahtchev, K.L.; Batovska, D.I.; Parushev, S.P.; Ubiyvovk, V.M.; Sibirny, A.A. Antifungal activity of chalcones: A mechanistic study using various yeast strains. Eur. J. Med. Chem. 2008, 43, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Detsi, A.; Majdalani, M.; Kontogiorgis, C.A.; Hadjipavlou-Litina, D.; Kefalas, P. Natural and synthetic 2′-hydroxy-chalcones and aurones: Synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorg. Med. Chem. 2009, 17, 8073–8085. [Google Scholar] [CrossRef] [PubMed]
- Bandgar, B.P.; Gawande, S.S.; Bodade, R.G.; Totre, J.V.; Khobragade, C.N. Synthesis and biological evaluation of simple methoxylated chalcones as anticancer, anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. 2010, 18, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Holla, B.S.; Akberali, P.M.; Shivananda, M.K. Studies on arylfuran derivatives: Part X. Synthesis and antibacterial properties of arylfuryl-Δ2-pyrazolines. Il Farmaco 2000, 55, 256–263. [Google Scholar] [CrossRef]
- Dawane, B.S.; Konda, S.G.; Mandawad, G.G.; Shaikh, B.M. Poly(ethylene glycol) (PEG-400) as an alternative reaction solvent for the synthesis of some new 1-(4-(4′-chlorophenyl)-2-thiazolyl)-3-aryl-5-(2-butyl-4-chloro-1H-imidazol-5yl)-2-pyrazolines and their in vitro antimicrobial evaluation. Eur. J. Med. Chem. 2010, 45, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Budakoti, A.; Bhat, A.R.; Athar, F.; Azam, A. Syntheses and evaluation of 3-(3-bromo phenyl)-5-phenyl-1-(thiazolo [4,5-b] quinoxaline-2-yl)-2-pyrazoline derivatives. Eur. J. Med. Chem. 2008, 43, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Khode, S.; Maddi, V.; Aragade, P.; Palkar, M.; Ronad, P.K.; Mamledesai, S.; Thippeswamy, A.H.M.; Satyanarayana, D. Synthesis and pharmacological evaluation of a novel series of 5-(substituted)aryl-3-(3-coumarinyl)-1-phenyl-2-pyrazolines as novel anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2009, 44, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).