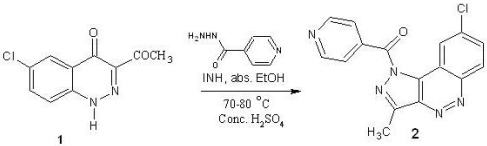
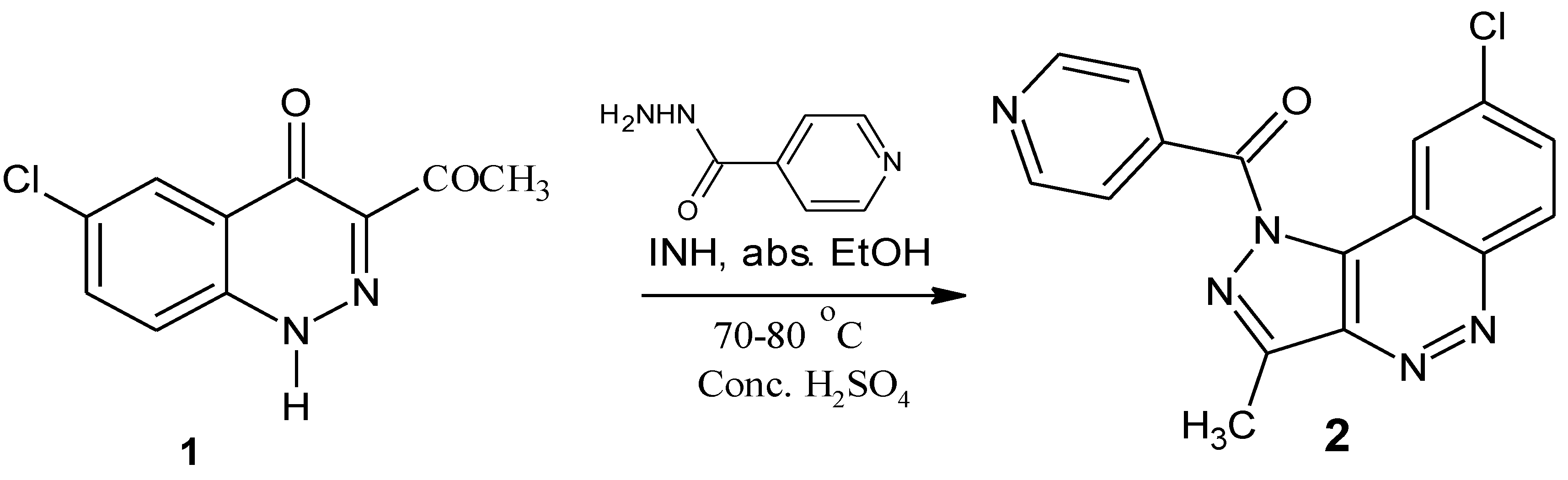
(8-Chloro-3-methyl-1H-pyrazolo[4,3-c]cinnolin-1-yl) (pyridin-4-yl)methanone
Abstract
:Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Narayana, B.; Vijayaraj, K.K.; Ashalatha, B.V.; Kumari, S.N. Antibacterial and antifungal studies on some new acetylcinnolines and cinnolinyl thiazole derivatives. Indian J. Chem. 2006, 45B, 1704–1709. [Google Scholar] [CrossRef]
- Schatz, F.; Wagner-Jauregg, T. Synthesis of substituted 1, 2-malonyl-1,2-dihydrocinnolin (1,3-Dioxo-2,3-dihydro-1H-pyrazolo[1,2-a]cinnoline) with anti-inflammatory properties. Helv. Chim. Acta 1968, 51, 1919–1931. [Google Scholar] [CrossRef] [PubMed]
- Barraja, P.; Diana, P.; Lauria, A.; Passannanti, A.; Almerico, A.M.; Minnei, C.; Longu, S.; Congiu, D.; Musiu, C.; La Colla, P. Indolo[3,2-c]cinnolines with antiproliferative, antifungal, and antibacterial activity. Bioorg. Med. Chem. 1999, 7, 1591–1596. [Google Scholar] [CrossRef]
- Keneford, J.R.; Simpson, J.C.E. Synthetic antimalarials. Part XX. cinnolines. Part XIII. Synthesis and antimalarial action of 4-aminoalkylaminocinnolines. J. Chem. Soc. 1947, 917–920. [Google Scholar] [CrossRef]
- Alvarado, M.; Barcelo, M.; Carro, L.; Masaguer, C.F.; Ravina, E. Synthesis and biological evaluation of new quinazoline and cinnoline derivatives as potential atypical antipsychotics. Chem. Biodivers. 2006, 3, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bawa, S.; Drabu, S. N-[(2-chloro-6-mthylquinolin-3-yl)methyl]aniline. Molbank 2009, 2009, M618. [Google Scholar] [CrossRef]
- Vingkar, S.L.; Bobade, A.S.; Khadse, B.G. Synthesis and antimicrobial activity of 3-2(alkyl/aryl,4-substituted thiozolo)-6-fluorocinnoline-4-ones. Indian J. Chem. 1993, 32B, 1281–1284. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bawa, S.; Kumar, R.; Chawla, G.; Kumar, S.; Mishra, R. (8-Chloro-3-methyl-1H-pyrazolo[4,3-c]cinnolin-1-yl) (pyridin-4-yl)methanone. Molbank 2010, 2010, M688. https://doi.org/10.3390/M688
Bawa S, Kumar R, Chawla G, Kumar S, Mishra R. (8-Chloro-3-methyl-1H-pyrazolo[4,3-c]cinnolin-1-yl) (pyridin-4-yl)methanone. Molbank. 2010; 2010(2):M688. https://doi.org/10.3390/M688
Chicago/Turabian StyleBawa, Sandhya, Rajiv Kumar, Gita Chawla, Suresh Kumar, and Ravinesh Mishra. 2010. "(8-Chloro-3-methyl-1H-pyrazolo[4,3-c]cinnolin-1-yl) (pyridin-4-yl)methanone" Molbank 2010, no. 2: M688. https://doi.org/10.3390/M688
APA StyleBawa, S., Kumar, R., Chawla, G., Kumar, S., & Mishra, R. (2010). (8-Chloro-3-methyl-1H-pyrazolo[4,3-c]cinnolin-1-yl) (pyridin-4-yl)methanone. Molbank, 2010(2), M688. https://doi.org/10.3390/M688