Abstract
A one-pot, two-step method has been developed for the synthesis of ethyl 2-[(Z)-2-(4-cyanophenyl)-2-hydroxyvinyl]-4-(4-methoxyphenyl)-6-methyl-1,4-dihydro-pyrimidine-5-carboxylate, including a sulfide contraction step utilizing solution and solid phase synthesis.
Nonplanar 3,4-dihydropyrimidin-2(1H)-one derivatives (DHPMs) are attractive molecules for drug research because of their known multifaceted pharmacological profiles. Introduction of DHPMs resulted in the discovery of new kinds of calcium channel modulators [1], hepatitis B virus replication inhibitors [2], mitotic kinesin inhibitors [3] and α1a-adrenergic receptor antagonists [4]. It seems to be reasonable that DHPMs are privileged structures for drug research and modifications around this motif are of considerable importance.
Due to our continuous efforts in search of new methods for modifying privileged hetreocycles, we report herein the synthesis of ethyl 2-[(Z)-2-(4-cyanophenyl)-2-hydroxyvinyl]-4-(4-methoxyphenyl)-6-methyl-1,4-dihydropyrimidine-5-carboxylate (3) from ethyl 4-(4-methoxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (1) in a one-pot, two-step synthesis (Scheme 1). The reaction sequence includes the S-alkylation of DHPM 1 by the α-bromoketone 2. The resulting intermediate undergoes in the presence of triphenylphosphine an Eschenmoser sulfide contraction to yield the desired product 3 [5]. Separation of the arising triphenylphosphine sulfide from the reaction mixture can be done by column chromatography, but is not an easy task. To prevent this costly separation procedure, we chose the application of polymer-bound triphenylphosphine. The reaction selectivity and yields of both phosphine agents are comparable. The presented synthetic method is well suited for a diversity-oriented synthesis strategy, using differently substituted Biginelli compounds and various α-bromoketones.
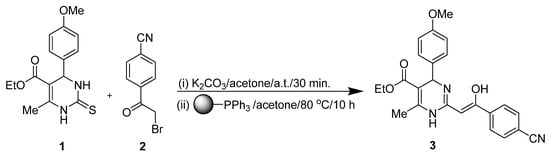
Scheme 1.
DHMP 1 alkylation/sulfide contraction sequence yielding product 3.
The title compound 3 has been fully characterized by 1H- and 13C-NMR, IR, Raman and ESI-TOF MS analysis. It was observed that the 1H NMR spectra of 3 show a duplicate set of some signals (approximately 3:1 ratio) due to different conformers of 3 in solution. No tautomeric form of 3 was observed in the NMR experiments. The 1H NMR as well as the 13C NMR did not show appropriate signals for the expected aliphatic CH2 group of a keto tautomer.
Experimental Procedure
To a suspension of ethyl 4-(4-methoxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate 1 (110 mg, 0.36 mmol, 1 equiv) in acetone (2 mL), powdered K2CO3 (75 mg, 0.54 mmol, 1.5 equiv) and 2-bromo-4`-cyanoacetophenone 2 (96 mg, 0.43 mmol, 1.2 equiv) were added subsequently. The reaction mixture was vigorously shaken at ambient temperature in a 4 mL capped glass vial, using an automatic shaker. A colour change of the solution from light yellow to dark brown took place. After shaking at ambient temperature under TLC monitoring (ethyl acatate/hexane 1:1, Rf: 0.3), complete conversion of 1 into the S-alkylated intermediate was observed after 30 min. Polymer-bound triphenylphosphine (0.54 mmol) (polystyrene, 2% DVB, 3 mmol/g, Fluka) was added in one portion, and the reaction temperature was raised to 80 oC. Shaking of the suspension was continued for additional 10 h to complete the sulfide contraction reaction. For work-up, the polymer was filtered off and washed with approx. 5 mL of ethyl acetate. The collected filtrates were concentrated under reduced pressure and subjected to flash chromatography (Silica-60, 0.06–0.20 mm, ethyl acetat/hexane 20% vol./vol.) to obtain pure viscous yellow product as an approx. 3:1 inseparable mixture of isomers in 65% (97 mg) yield.
HR-MS (ESI-Q-TOF) calcd. for C24H24N3O4 [MH]+: 418.1761 found [MH]+: 418.1769.
UV-Vis (CH3CN), λ (nm): 374, 282, 244, 193.
IR (KBr, cm-1): 2977 (C-H aromatic), 2227 (-C≡N), 1700 (C=O), 1630 (C=C), 1573, 1532, 1509, 1476, 1368, 1328, 1244, 1203, 1172, 1092, 1058, 1031.
Raman (powder, ATR, cm-1): 2229, 1649, 1605, 1502, 1382, 1300, 1222, 1177, 1111, 872, 763.
1H NMR (300 MHz, CDCl3), δ (ppm): 1.13, 1.12 (t, 3H, J = 6.0 Hz, -OCH2CH3), 2.41, 2.33 (s, 3H, CH3), 3.71, 3.68 (s, 3H, OCH3), 4.03, 4.05 (q, 2H, J = 6.0 Hz, -OCH2CH3), 5.22 (s, 1H, CH), 5.28 (s, 1H, CH), 5.63, 5.38 (1H, D2O exchangeable, OH), 6.77, 6.74 (d, 2H, J = 9.0 Hz, ArH), 7.17, 7.13 (d, 2H, J = 9.0 Hz, ArH), 7.58, 7.54 (d, 2H, J = 9.0 Hz, ArH), 7.76, 7.72 (d, 2H, J = 9.0 Hz, ArH), 12.72, 11.41 (br, 1H, D2O exchangeable, N1-H). The second set of 1H NMR signals (observed for the minor isomeric form) is italicized.
13C NMR (75 MHz, CDCl3), δ (ppm): 14.2, 19.3, 52.8, 55.3, 60.3, 79.0, 103.7, 114.2, 118.6, 127.1, 128.0, 132.1, 135.8, 143.2, 144.3, 155.0, 159.5, 165.3, 183.9.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
Financial support by the Federal Ministry of Education and Research, Germany (BMBF) (FKZ 16SV3701) and by the Thuringian Ministry of Culture (FKZ 03ZIK062, FZK 03ZIK465) is gratefully acknowledged. The support from S. Günther and K. Risch for spectroscopic measurements is gratefully acknowledged.
References and Notes
- Kappe, C.O. Biologically active dihydripyrimidones of the Biginelli-Type. A literature survey. Eur. J. Med. Chem. 2000, 35, 1043–1052. [Google Scholar] [CrossRef]
- Deres, K.; Schroeder, C.H.; Paessens, A.; Goldmann, S.; Hacker, H.J.; Weber, O.; Kramer, T.; Niewoehner, U.; Pleiss, U.; Stoltefuss, J.; Graef, E.; Koletzki, D.; Masantschek, R.N.A.; Reimann, A.; Jaeger, R.; Grob, R.; Beckermann, B.; Schlemmer, K.H.; Haebich, D.; Ruebsamen-Waigmann, H. Inhibition of hepatitis B virus replication by drug-induced depletion of nucleocapsids. Science 2003, 299, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Mayer, T.U.; Kapoor, T.M.; Haggarty, S.J.; King, R.W.; Schreiber, S.L.; Mitchison, T.J. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 1999, 286, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Barrow, J.C.; Nantermet, P.G.; Selnick, H.G.; Glass, K.L.; Rittle, K.E.; Gilbert, K.F.; Steele, T.G.; Homnick, C.F.; Freidinger, R.M.; Ransom, R.W.; Kling, P.; Reiss, D.; Broten, T.P.; Schorn, T.W.; Chang, R.S.L.; O’Malley, S.S.; Olah, T.V.; Ellis, J.D.; Barrish, A.; Kassahun, K.; Leppert, P.; Nagarathnam, D.; Forray, C. In vitro and in vivo evaluation of dihydropyrimidinone C-5 amides as potent and selective α1A receptor antagonists for the treatment of benign prostatic hyperplasia. J. Med. Chem. 2000, 43, 2703–2718. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Schober, A.; Gebinoga, M.; Groß, G.A. Facile conversion of Biginelli 3,4-dihydropyrimidin-2(1H)-thiones to 2-(2-hydroxy-2-arylvinyl)dihydropyrimidines via Eschenmoser coupling. Tetrahedron Lett. 2009, 50, 1838–1843. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).