Abstract
A bis(chloroethyl)amine-containing side chain is attached to an oxonaphthalene-annelated pyrrole in expectation of DNA alkylating properties. The cytotoxicity is evaluated against two cell lines, KB-31 and KB-8511, respectively.
Introduction
Nitrogen mustards were the first clinically effective cancer therapeutic agents. Chlorambucil and melphalan are two of numerous aromatic derivatives of compounds with a nitrogen mustard moiety that have been synthesized. They have been clinical agents for many years and remain in common use at the present time. The cytotoxic effects are based on the highly active aziridinium cation intermediates arising from the bis(2-chloroethyl)amine moiety [1]. In continuation of our department’s previous studies in the field of antitumor agents [2,3,4,5,6,7,8,9], we are reporting in this paper the synthesis of the oxonaphthalene-annelated pyrrole 3 with an attached side chain containing a bis(2-chloroethyl)¬amine group. The cytotoxic activity of 3 was evaluated.
Results and Discussion
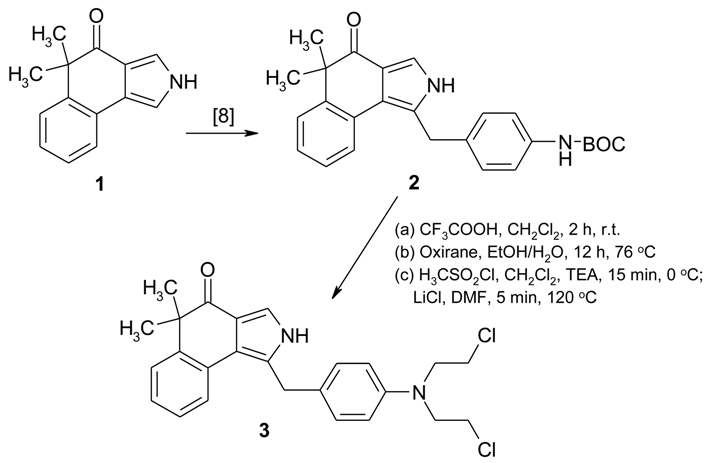
As previously published, N-alkylation of 1 [10,11] with BOC-protected 4-aminobenzyl bromide with NaH in THF selectively afforded 2 [8], the following deprotection with trifluoroacetic acid [12] furnished the amine which was treated with ethylene oxide [4]. The resulting diol was converted via its mesylate and subsequent reaction with LiCl [13] into the target compound 3. The biological activity of 3 was tested against two cancer cell lines, KB-31 and KB-8511, respectively. KB-31 is a drug sensitive human epidermoid cell line, whereas KB-8511 is a multi-drug resistant subline, typically over¬expressing P-glycoprotein. The IC50[μM] values of 3 are >2.815 (KB-31) and 2.496 (KB-8511), respectively (for experimental details, see [14,15]).

Experimental
1-[4-[Bis(2-chloroethyl)amino]benzyl]-5,5-dimethyl-2,5-dihydro-4H-benzo[e]isoindol-4-one (3)
(a) To a solution of 2 [8] (0.7 g, 1.68 mmol) in 7.7 mL of dry CH2Cl2 were added dropwise under argon 1.53 mL (19.85 mmol) of triflouroacetic acid. After stirring for 2 h at room temperature, the reaction mixture was concentrated under vacuum and the residue was dissolved in ethyl acetate. The organic phase was washed with aqueous NaHCO3-solution and brine, dried (Na2SO4) and concentrated.
(b) The resulting crude product was purified by column chromatography (aluminium oxide, light petro¬leum/ethyl acetate, 60/40) to afford a solid residue which was dissolved in 25 mL of EtOH/H2O (3/1). 0.55 mL (0.61 g, 1.40 mmol) of oxirane were added to the reaction mixture at 0 °C. After heating for 12 h under reflux, the reaction mixture was concentrated under vacuum and the residue was dissolved in ethyl acetate. The organic phase was dried (Na2SO4) and concentrated. The resulting crude product was used for the next reaction step without further purification.
(c) To a solution of the obtained crude product in 4.8 mL of dry CH2Cl2 and 0.4 mL of triethylamine were added dropwise under argon 0.20 mL (2.55 mmol) of methanesulfonyl chloride. After stirring for 15 min, the reaction mixture was washed with H2O, dried (Na2SO4) and concentrated under vacuum, The residue was dissolved in 4.5 mL of DMF and, after addition of 0.8 g LiCl (18.90 mmol; dried over P2O5 at 100 °C for 12 h), the reaction mixture was heated for 5 min at 120 °C. The cooled reaction mixture was diluted with H2O and extracted with ethyl acetate. The combined organic layers were washed with H2O, dried (Na2SO4) and concentrated. The crude product was purified by column chromatography (silica gel, light petroleum/ethyl acetate 60/40 + 0.3% TEA) to afford 298 mg (40%) of 3. M.p. 67–69 °C (light petroleum/ethyl acetate). IR (KBr): 3236, 1644, 1519, 1353, 1179 cm-1. MS (EI, 70 eV) m/z: 442 (M++2, 14%), 440 (M+, 22), 391 (100), 350 (46), 167 (46), 118 (44), 106 (42). 1H NMR (CDCl3, 200 MHz) δ = 9.12 (sbr, 1H, NH), 7.66 (m, 1H, 9-H), 7.47 (m, 1H, 6-H), 7.34 (d, J = 3.0 Hz, 1H, 3-H), 7.22 (m, 2H, 7-H, 8-H), 7.12 (d, J = 8.5 Hz, 2H, 2’-H, 6’-H), 6.62 (d, J = 8.5 Hz, 2H, 3’-H, 5’-H), 4.26 (s, 2H, C-1-CH2), 3.68 (m, 4H, 2 × CH2N), 3.59 (m, 4H, 2 × CH2Cl), 1.51 (s, 6H, (CH3)2). 13C NMR (CDCl3, 50 MHz) δ = 199.8 (C-4), 144.9 (C-4’), 144.1 (C-5a), 130.0 (C-2’, C-6’), 128.4/126.7/125.9 (C-1’, C-9a, C-9b), 127.1 (C-6), 126.3 (C-7), 125.9 (C-8), 123.5 (C-9), 119.5/118.7 (C-1, C-3a), 118.98 (C-3), 112.4 (C-3’, C-5’), 53.4 (2 × CH2N), 47.8 (C-5), 40.4 (2 × CH2Cl), 33.0 (CH2-C-1), 28.2 ((CH3)2). HRMS calc. for C25H26N2OCl2: 440.1422. Found: 440.1417.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgement
We are indepted to Novartis AG (Vienna, Austria) for the evaluation of the cytotoxic activity.
References and Notes
- Montgomery, J.A. In Cancer Chemotherapeutic Agents, ACS Pro¬fessional Reference Book; Foye, W.O., Ed.; American Chemical Society: Washington, DC, USA, 1995; pp. 111–121. [Google Scholar]
- Pongprom, N.; Mueller, G.; Schmidt, P.; Holzer, W.; Spreitzer, H. Carbinol derivatives of azanaphthoquinone annelated pyrrols. Monatsh. Chem. 2009, 140, 309–313. [Google Scholar] [CrossRef]
- Shanab, K.; Pongprom, N.; Wulz, E.; Holzer, W.; Spreitzer, H.; Schmidt, P.; Aicher, B.; Mueller, G.; Günther, E. Synthesis and biological evaluation of novel cytotoxic azanaphthoquinone annelated pyrrolo oximes. Bioorg. Med. Chem. Lett. 2007, 17, 6091–6095. [Google Scholar] [CrossRef] [PubMed]
- Spreitzer, H.; Puschmann, C. Dual function antitumor agents based on bioreduction and DNA-alkylation. Monatsh. Chem. 2007, 138, 517–522. [Google Scholar] [CrossRef]
- Haider, N.; Sotelo, E. 1,5-Dimethyl-6H-pyridazino[4,5-b]carbazole, a 3-Aza bioisoster of the antitumor alkaloid olivacine. Chem. Pharm. Bull. 2002, 50, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Kabicher, T.; Käferböck, J.; Plenk, A. Synthesis and in-vitro antitumor activity of 1-[3-(indol-1-yl)prop-1-yn-1-yl]phthalazines and related compounds. Molecules 2007, 12, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Jbara, R.; Käferböck, J.; Traar, U. Synthesis of tetra- and pentacyclic carbazole-fused imides as potential antitumor agents. Arkivoc 2009, 38–47. [Google Scholar]
- Spreitzer, H.; Puschmann, C. Regioselective alkylation of an oxonaphthalene-annelated pyrrol system. Molbank 2009, 2009, M619. [Google Scholar] [CrossRef]
- Spreitzer, H.; Puschmann, C. Synthesis of cytotoxic oxonaphthalene-pyrroles I. Molbank 2010, 2010, M651. [Google Scholar] [CrossRef]
- Spreitzer, H.; Holzer, W.; Puschmann, C.; Pichler, A.; Kogard, A.; Tschetschkowitsch, K.; Heinze, T.; Bauer, S.; Shabaz, N. Synthesis and NMR-investigation of annelated pyrrole derivatives. Heterocycles 1997, 45, 1989–1997. [Google Scholar] [CrossRef]
- Spreitzer, H.; Holzer, W.; Fülep, G.; Puschmann, C. N-substituted 5,5-dimethyl-2,5-dihydro-4H-isoindol-4-ones: Synthesis and NMR-investigation. Heterocycles 1996, 43, 1911–1922. [Google Scholar] [CrossRef]
- Brown, F.J.; Bernstein, P.R.; Cronk, L.A.; Dosset, D.L.; Hebbel, K.C.; Maduskuie, T.P., Jr.; Shapiro, H.S.; Vacek, E.P.; Yee, Y.K.; Willard, A.K.; Krell, R.D.; Snyder, D.W. Hydroxyacetophenone-derived antagonists of the peptidoleukotrienes. J. Med. Chem. 1989, 32, 807–826. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.D.; Wilson, W.R.; Anderson, R.F.; Boyd, M.; Denny, W.A. Hypoxia-selective antitumor agents. 14. Synthesis and hypoxic cell cytotoxicity of regioisomers of the hypoxia-selective cytotoxin 5-[N,N-Bis(2-chloroethyl)amino]-2,4-dinitrobenzamide. J. Med. Chem. 1996, 39, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Regenass, U.; Fabbro, D.; Alteri, E.; Rösel, J.; Müller, M.; Caravatti, G.; Matter, A. A derivative of staurosporine (CGP 41 251) shows selectivity for protein kinase C inhibition and in vitro anti-proliferative as well as in vivo anti-tumor activity. Int. J. Cancer 1989, 43, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Scarpelli, R.; Bollbuck, B.; Werschkun, B.; Pereira, M.M.; Wartmann, M.; Altmann, K.H.; Zaharevitz, D.; Guscio, R.; Giannakakou, P. Chemical synthesis and biological properties of pyridine epothilones. Chem. Biol. 2000, 7, 593–599. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).