Abstract
Manganese complex of 5,10,15,20-tetra(N-ethyl-3-carbazolyl) porphyrin was synthesized and characterized by electronic absorption spectrophotometry and cyclic voltammetry. The spectral data were in agreement with the proposed structure. The manganese complex exhibited a shift in the Soret band in comparison to the non-metallated porphyrin and the extinction coefficient for the Soret band was on the order of 105 cm-1M-1. Trends observed in the oxidation and reduction potentials were consistent with the nature of the porphyrin. That is, the electron donating group in 5,10,15,20-tetra(N-ethyl-3-carbazolyl) porphyrin enhances oxidation and inhibits reduction.
Introduction
Porphyrins are of interest in many areas of study including photodynamic cancer therapy, optical data storage, and solar energy. The focus of this paper is to report the synthesis, electronic absorption spectroscopy, and cyclic voltammetry of the manganese complex of 5,10,15,20-tetra(N-ethyl-3-carbazolyl) porphyrin, TECP.
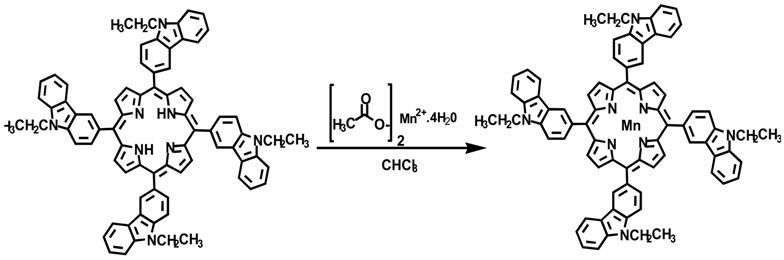

Experimental Section
Chemicals and Reagents
Reagents and solvents used in the synthesis and metallation of TECP, NMR measurements, and UV-vis were purchased from Aldrich Chemical Company and used as received. Methylene chloride used in cyclic voltammetry experiments was distilled over calcium hydride before use. Tetrabutylammonium tetrafluoroborate was purchased from Fisher Scientific Company and was used as received.
Instrumentation
Ultraviolet-visible (UV-vis) absorption spectra were recorded on a Hewlett Packard 8451A spectrophotometer. Cyclic voltammograms (CV) were recorded using a computer linked EG&G Princeton Applied Research Potentiostat/Galvanostat Model 273 equipped with an IR compensator and model 270 Electrochemical software. The CVs were obtained in a four-necked cell with a platinum working electrode, a platinum wire counter electrode, and a saturated calomel electrode (SCE) as the reference electrode. A capacitor (0.1 μF), was connected between the counter electrode and the reference electrode to reduce background noise. The porphyrin solutions were 1 × 10-3 M in 0.1 M methylene chloride solutions of tetrabutylammonium tetrafluoroborate. Potentials were reported versus a S.C.E. and not corrected for liquid junction potentials. Deaeration of the solution was achieved by passing a stream of nitrogen through the solution. The platinum working electrode was cleaned with solvent, using a lint free wipe, between each run and polished periodically.
Synthesis
5,10,15,20-Tetra(N-ethyl-3-carbazolyl) Porphyrin: TECP was synthesized as reported previously [1]. The structure was confirmed using 1H NMR, 13C NMR, UV-Vis and high resolution mass spectrometry. UV (CHCl3) [λ,nm (ε, cm-1M-1)]: 433 (2.8 × 105), 523 (1.3 × 104), 562.5 (1.1 × 104), 654.5 (5.6 × 103). 1H NMR (360 MHz, CDCl3), δ (ppm): 8.97 (s, 4H), 8.89 (s, 8H), 8.37 (d, J = 8.1 Hz, 4H), 8.19 (d, J = 7.8 Hz, 4H), 7.75 (d, J = 8.2 Hz, 4H), 7.58 (m, 8H), 7.28 (t, J = 7.8 Hz, 4H), 4.63 (q, J = 7.1 Hz, 8H), 1.68 (t, J = 7.0, 12H), –2.4 (s, 2H). 1H NMR (360 MHz, Acetone-d6), δ (ppm): 9.03 (s, 4H), 8.93 (s, 8H), 8.40 (d, J = 8.4 Hz, 4H), 8.32 (d, J = 7.6 Hz, 4H), 7.99 (d, J = 8.4 Hz, 4H), 7.76 (d, J = 8.3 Hz, 4H), 7.58 (t, J = 7.7 Hz, 4H), 7.27 (t, J = 7.5 Hz, 4H), 4.76 (q, J = 7.1 Hz, 8H), 1.65 (t, J = 7.0 Hz, 12H), –2.4 (s, 2H). HRMS-FAB (m/z): calcd for C76H59N8: 1083.486 a.m.u.; observed: 1083.487 a.m.u.
Manganese 5,10,15,20-Tetra(N-ethyl-3-carbazolyl) Porphyrin: The manganese 5,10,15,20-tetra(N-ethyl-3-carbazolyl) porphyrin was prepared using chromatographed TECP (0.130 g, 1.20 × 10-4 mol) in approximately 250 mL of boiling chloroform. The solution turned very dark, almost black, in color as the porphyrin dissolved in the chloroform. Once the porphyrin was dissolved, 3 mL of a saturated solution of manganese(II) acetate tetrahydrate in methanol was added. An additional 3 mL was added after 48 h as the reaction was not complete as indicated with UV-vis. The solution was refluxed for a total of 63.5 h. The shift in the λmax to 484 nm indicated the formation of manganese 5,10,15,20-tetra(N-ethyl-3-carbazolyl) porphyrin. The reaction mixture was evaporated to dryness, washed with 1 L of distilled water, and filtered giving 1.26 g of crude Mn(III)TECP. The Mn(III)TECP was purified using a silica gel column with CHCl3 as the eluent until the unreacted TECP came off the column in a red band. Then, a 9:1 mixture of chloroform and methanol was used to elute the MnTECP which came off in a green band. For 0.1686 g crude compound chromatographed, 0.0183 g of pure compound was obtained, giving an overall yield of 95.3%. UV-vis Data [λ, nm (ε, cm-1M-1)], CHCl3 484 (9.55 × 104), 630 (1.03 × 104).
Results and Discussion
In the electronic absorption spectra of the metallated porphyrin, a shift in the Soret band and the disappearance of at least one of the Q bands is expected and observed. This disappearance is due to the increased symmetry acquired by the porphyrin with introduction of a metal ion to the ring. All of the extinction coefficients for the Soret band are on the order of 105 cm-1M-1.
The reduction potential of MnTECP in CH2Cl2, –0.255 V vs. S.C.E., agrees with literature values for manganese (III) tetraphenyl porphyrin. Kadish reported a reduction potential for MnTPPCl of –0.25 V vs. S.C.E. in DMSO [2]. Many other manganese porphyrins have been studied and give reduction potentials that are in agreement with the values given here [2–5]. A one-electron oxidation is observed at +1.036 V vs. S.C.E. for MnTECP. Due to the electron withdrawing groups on MnTECP it should be easier to oxidize than MnTPPCl. This is confirmed as MnTPPCl is reported to undergo oxidation at +1.14 V vs. S.C.E. [2]. Studies of other manganese porphyrins give similar results [2,3].
Conclusion
All data observed for manganese complex of TECP follow trends expected when compared to the TPP complexes.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
This work was financially supported by the Research and Special Projects Committee of the University of Montevallo and a graduate fellowship at the University of Alabama funded in part by the Sponsors of the Materials for Information Technology Center. We would like to thank Ken Belmore, the Nuclear Magnetic Resonance specialist at the University of Alabama, for running the NMR of our base porphyrin.
References and Notes
- Tidwell, C.P.; Alexander, L.A.; Fondren, L.D.; Belmore, K.; Nikles, D.E. Synthesis and characterization of 5,10,15,20-tetra(N-ethyl-3-carbazoyl) porphyrin. Indian J. Chem. Sect. B 2007, 46B, 1658–1665. [Google Scholar]
- Kelly, S.L.; Kadish, K.M. Counterion and solvent effects on the electrode reactions of manganese porphyrins. Inorg. Chem. 1982, 21, 3631–3639. [Google Scholar] [CrossRef]
- Boucher, L.J.; Garber, H.K. Manganese porphyrin complexes. IV. Reduction of manganese porphyrins. Inorg. Chem. 1970, 9, 2644–2649. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
