Abstract
The title compound was prepared by treatment of N,N’-di(4-picolylamino)ethane with N,N-dimethylformamide dimethylacetal in toluene and it was characterized by elemental analysis, 1H NMR and 13C NMR.
Introduction
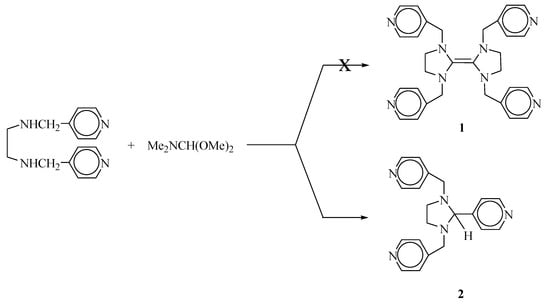
Olefins with four electron-donating substituents react as nucleophiles and are referred to as electron-rich olefins [1,2]. These compounds have been used as strong reducing agents, precursors to carbene complexes, effective formylating agents for proton-active compounds, catalysts for benzoin-type C-C coupling reactions and chemoluminescent materials [3,4,5,6,7]. Carbene complexes, formed by treatment of an electron-rich olefin with metal, are air-stable and have been used for several catalytic applications including olefin metathesis, hydrogenation and hydrosilylation reactions [8,9,10]. Symmetric electron-rich olefins are generally prepared from an N,N’-disubstituted 1,2-diaminoethane with N,N-dimethylformamide dimethylacetal in an inert atmosphere [11]. Unsymmetric and bridged electron-rich olefins are prepared by a convenient salt elimination procedure [12]. The compound 1 was not obtained using the acetal procedure, and if formed, it would spontaneously rearrange to the compound 2. This thermal amino-Claisen rearrangement was believed to be [3,3]-sigmatropic [13,14]. We report here on the synthesis and characterization of 2-(4-pyridyl)-1,3-di(4-picolyl)imidazolidine.
Experimental
Reaction for the preparation of 2-(4-pyridyl)-1,3-di(4-picolyl)imidazolidine was carried out under argon using standard Schlenk-type flasks. Solvents were dried using standard procedures and were distilled prior to use. 1H and 13C NMR spectra were recorded in DMSO-d6 using a Bruker AC300P FT spectrometer operating at 300.13 MHz (1H) and 75.47 MHz (13C). Chemical shifts (δ) are given in ppm relative to TMS and coupling constants (J) in Hertz. The melting point was measured in open capillary tubes with an Electrothermal-9200 melting point apparatus and it is uncorrected. Elemental analyses was performed at TUBITAK Microlab (Ankara, Turkey).
Synthesis of 2-(4-pyridyl)-1,3-di(4-picolyl)imidazolidine (2)
A solution of N,N’-di(4-picolylamino)ethane (1.87 g; 8.5 mmol) and N,N-dimethylformamide dimethyl acetal (1.10 g; 9.27 mmol) in toluene (15 mL) was heated under reflux for 4 h at 90 °C. The reaction mixture was then heated at 110 °C under distillation conditions, allowing the produced dimethylamine and methanol to escape. Hexane (10 mL) was added and a white solid precipitaded. The crude product was filtered off and washed with hexane (2 × 10 mL). The precipitate was then crystallized from toluene/hexane (10:10 mL). Yield: 0.93 g, 73%, m.p.: 125 oC.
1H NMR (CDCl3) δ: 3.3 (s, 1H, 2-CH), 1.71 and 2.52 (m, 4H, NCH2CH2N), 2.53 and 3.03 (m, 4H, CH2C5H4N), 6.55 and 8.26 (d, 4H, J = 5.6 Hz, CH2C5H4N and m, 4H, CH2C5H4N), 6.88 and 8.25 (m, 4H, C5H4N).
13C NMR(CDCI3) δ: 87.3 (2-CH), 50.8 (NCH2CH2N), 55.7 (CH2C5H4N), 123.2, 147.2 and 150.2 (CH2C5H4N), 124.1, 148.9 and 150.5 (C5H4N).
Anal. Calcd. for C20H21N5: C, 72.50; H, 6.34; N, 21.14. Found C, 72.87; H, 6.12; N, 21.38.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Hoffman, R.W. Reactions of electron-rich olefins. Angew. Chem. Int. Ed. 1968, 7, 754–765. [Google Scholar] [CrossRef]
- Wiberg, N. Tetraaminoethylenes as strong electron donors. Angew. Chem. Int. Ed. 1968, 7, 766–779. [Google Scholar] [CrossRef]
- Bock, H.; Borrmann, H.; Havlas, Z.; Oberhammer, H.; Ruppert, K.; Simon, A. Structures of sterically overcrowded and chargeperturbed molecules. 12. tetrakis(dimethylamino) ethene an extremely electron-rich molecule with unusual structure both in the crystal and in the gas-phase. Angew. Chem. Int. Ed. Engl. 1991, 30, 1678–1681. [Google Scholar] [CrossRef]
- Cardin, D.J.; Çetinkaya, B.; Lappert, M.F.; Muir, L.J.M.; Muir, K.W. An electron-rich olefin as a source of co-ordinated carbene; Synthesis of trans-PtCl2[C(NPhCH2)2]PEt3. Chem. Commun. 1971, 400–401. [Google Scholar] [CrossRef]
- Cardin, D.J.; Çetinkaya, B.; Çetinkaya, E.; Lappert, M.F.; Muir, L.J.M.; Muir, K.W. Trans-/cis-Isomerism and isomerisation of Pd(II) and Pt(II) carben complexes. J. Organomet. Chem. 1972, 44, C59–62. [Google Scholar] [CrossRef]
- Wanzlick, H.; Schikora, E. Ein nucleophiles Carben. Chem. Ber. 1961, 94, 2389–2393. [Google Scholar] [CrossRef]
- Lappert, M.F.; Maskell, R.K. A new class of benzoin condensation catalyst, the bis-(1,3-dialkylimidazolidin-2-ylidenes). J. Chem. Soc., Chem. Commun. 1982, 580–581. [Google Scholar]
- Çetinkaya, B.; Demir, S.; Özdemir, İ.; Toupet, L.; Semeril, D.; Bruneau, C.; Dixneuf, P.H. First rutnenium complexes with a chelating arene carbene ligand as catalytic precursors for alkene metathesis and cycloisomerisation. New J. Chem. 2001, 25, 519–521. [Google Scholar]
- Yiğit, M.; Yiğit, B.; Özdemir, İ.; Çetinkaya, E.; Çetinkaya, B. Active ruthenium-(N-Heterocyclic Carbene) complexes for hydrogenation of ketones. Appl. Organometal. Chem. 2006, 20, 322–327. [Google Scholar] [CrossRef]
- Lappert, M.F.; Maskell, R.K. Carbene-transition-metal complexes as hydrosilylation catalysts. J. Organomet. Chem. 1984, 264, 217–228. [Google Scholar] [CrossRef]
- Winberg, H.E.; Carnahan, J.E.; Coffman, D.D.; Brown, M. Tetraaminoethylenes. J. Am. Chem. Soc. 1965, 87, 2055–2056. [Google Scholar] [CrossRef]
- Çetinkaya, E.; Hitchcock, P.B.; Jasim, H.A.; Lappert, M.F.; Sypropoulos, K. Synthesis and characterisation of unusual tetraaminoalkenes (Enetetramines). J. Chem. Soc., Perkin Trans. 1 1992, 561. [Google Scholar]
- Boldwin, J.E.; Walker, J.A. Competing [1,3]- and [3,3]-sigmatropic rearrangements of electron-rich olefins. J. Am. Chem. Soc. 1974, 96, 596–597. [Google Scholar] [CrossRef]
- Chamizo, J.A.; Lappert, M.F. [3,3]- and [1,3]-Sigmatropic amino-Claisen rearrangements of electron-rich alkenes. J. Org. Chem. 1989, 54, 4684–4686. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).