3-Methyl-4,5,6,7-tetrahydro-1-benzothiophene-2-carboxylic Acid
Abstract
:1. Introduction
2. Result and Discussions
3. Experimental
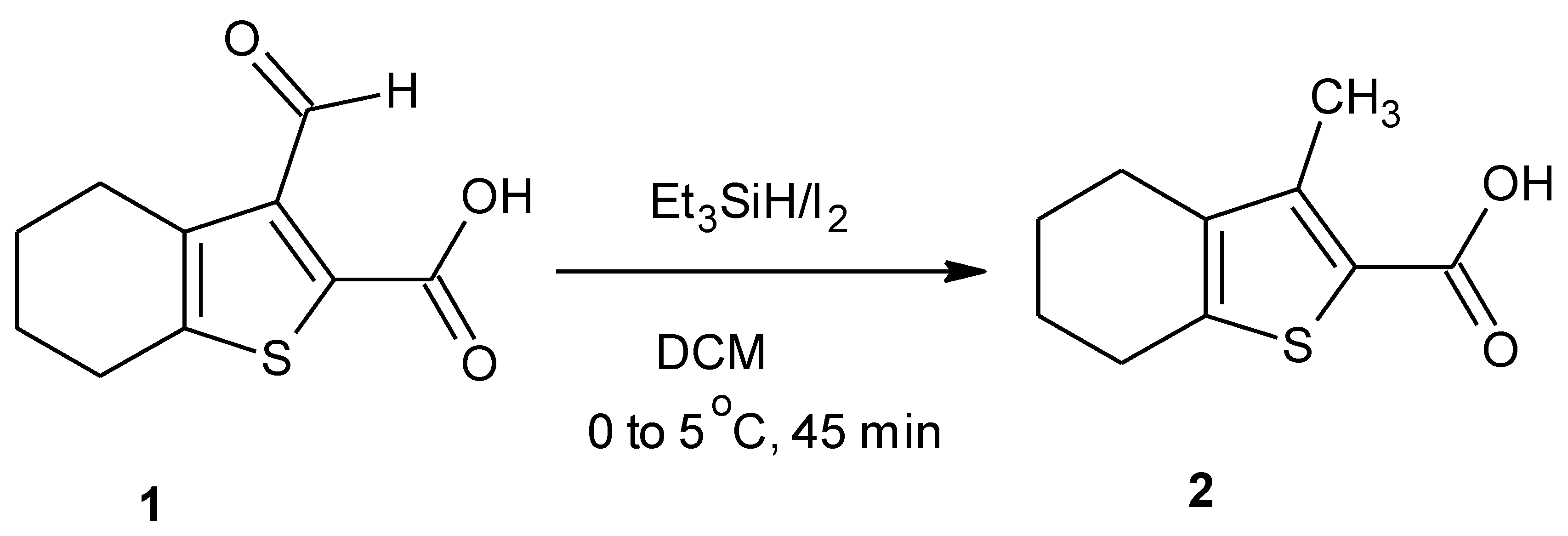
3-Methyl-4,5,6,7-tetrahydro-1-benzothiophene-2-carboxylic acid (2)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Lam, H.W.; Joensuu, P.M.A. Cu(I)-Catalysed reductive aldol cyclisation; Diastereo- and enatioselective synthesis of β-hydroxylactones. Org. Lett. 2005, 7, 4225–4228. [Google Scholar] [CrossRef] [PubMed]
- Semmelhack, F.M.; Raj, N.M. Stereoselective ketone reduction: Reduction of 4-tert-butylcyclohexanone by alkylsilane in the presence of rhodium (I) and rhodium (II) catalysts. J. Org. Chem. 1982, 47, 2467–2469. [Google Scholar] [CrossRef]
- Jesus, M.A.; Lecea, B.; Palomo, C. Reduction of carbonyl compounds promoted by silicon hydrides under the influence of trimethylsilyl based reagents. Can. J. Chem. 1986, 64, 2342–2347. [Google Scholar]
- Jesus, M.A.; Lecea, B.; Palomo, C. Synthesis of benzyl halides from aldehydes promoted by halosilanes and 1,1,3,3-tetrametheyldisiloxane. Tetrahedron Lett. 1984, 25, 1103–1104. [Google Scholar]
- Evans, D.A.; Fitch, D.M.; Smith, T.E.; Cee, V.J. Reduction of hemiacetals to cyclic ethers. J. Am. Chem. Soc. 2000, 12, 10033–10046. [Google Scholar] [CrossRef]
- Michael, P.D.; Donald, J.D.; Stephen, J.D.; Dale, A.K.; Abayomi, A.O.; Charles, T.W.; Steven, M.Z. Silane reduction in acidic media. III. Reductions of aldehydes and ketones to alcohols and alcohol derivatives. General synthesis of alcohols, symmetric ethers, carboxylate esters and acetamides. J. Org. Chem. 1974, 39, 2740–2747. [Google Scholar]
- Campos, P.J.; Garcia, G.; Rodriguez, M.A. A simple and versatile method for the hydroiodination of alkenes and alkynes using I2 and Et3SiH in the presence of copper(II). Tetrahedron Lett. 2002, 43, 6111–6112. [Google Scholar] [CrossRef]
- Yadav, J.S.; Subba Reddy, B.V.; Premalatha, K.; Swamy, T. First example of the activation of polymethylhydrosiloxane with molecular iodine: A facile synthesis of 3,6-dihydropyran derivatives. Tetrahedron Lett. 2005, 44, 2687–2690. [Google Scholar] [CrossRef]
- Adinolfi, M.; Iadonisi, A.; Ravida, A.; Schiattarella, M. Efficient and direct synthesis of saccharidic 1,2-ethylidines, orthoesters, and glycals from peracetylated sugars via the in situ generation of glycosyl iodides with I2/Et3SiH. Tetrahedron Lett. 2003, 44, 7863–7866. [Google Scholar] [CrossRef]
- Adinolfi, M.; Barone, G.; Iadonisi, A.; Schiattarella, M. Iodine/Triethylsilane as a convenient promoter system for the activation of disarmed glycals trichloro- and N-(phenyl)trifluoroacetimidates. Synlett 2002, 269. [Google Scholar] [CrossRef]
- Kumar, P.R.; Raju, S.; Goud, P.S.; Sailaja, M.; Sarma, M.R.; Reddy, G.O.; Kumar, M.P.; Reddy, V.V.; Suresh, T.; Hedge, P. Synthesis and biological evalution of thiophene[3,2-b]pyrrole derivatives as potential anti-inflammatory agents. Bioorg. Med. Chem. 2004, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.M.H.; Rahman, K.M.M.; Hossain, M.K.; Rahim, A.; Hossain, M.I.; Abu Naser, M. Synthesis and antimicrobial evaluation of some new thienopyrimidine derivatives. Act. Pharm. 2006, 56, 441–450. [Google Scholar]
- Algarsamy, V.; Meena, S.; Ramesh, K.V.; Soloman, V.R.; Thirumurugan, K.; Dhanabal, K.; Murugan, M. Synthesis, analgesic, anti-inflammatory, ulcerogenic index and antibacterial activities of novel 2-methylthio-3-substituted-5,6,7,8-tetrahydrobenzo(b)thieno[2,3-d]pyrimidine-4(3H)-ones. Eur. J. Med. Chem. 2006, 41, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jayaraman, S.R.; Sridharan, M.; Nagappan, R. 3-Methyl-4,5,6,7-tetrahydro-1-benzothiophene-2-carboxylic Acid. Molbank 2010, 2010, M648. https://doi.org/10.3390/M648
Jayaraman SR, Sridharan M, Nagappan R. 3-Methyl-4,5,6,7-tetrahydro-1-benzothiophene-2-carboxylic Acid. Molbank. 2010; 2010(1):M648. https://doi.org/10.3390/M648
Chicago/Turabian StyleJayaraman, Sembian Ruso, Madhavan Sridharan, and Rajendiran Nagappan. 2010. "3-Methyl-4,5,6,7-tetrahydro-1-benzothiophene-2-carboxylic Acid" Molbank 2010, no. 1: M648. https://doi.org/10.3390/M648
APA StyleJayaraman, S. R., Sridharan, M., & Nagappan, R. (2010). 3-Methyl-4,5,6,7-tetrahydro-1-benzothiophene-2-carboxylic Acid. Molbank, 2010(1), M648. https://doi.org/10.3390/M648



