5-(3-Nitrophenyl)-3-phenyl-4,5-dihydro-1H-pyrazole-1-carbaldehyde
Abstract
:1. Introduction
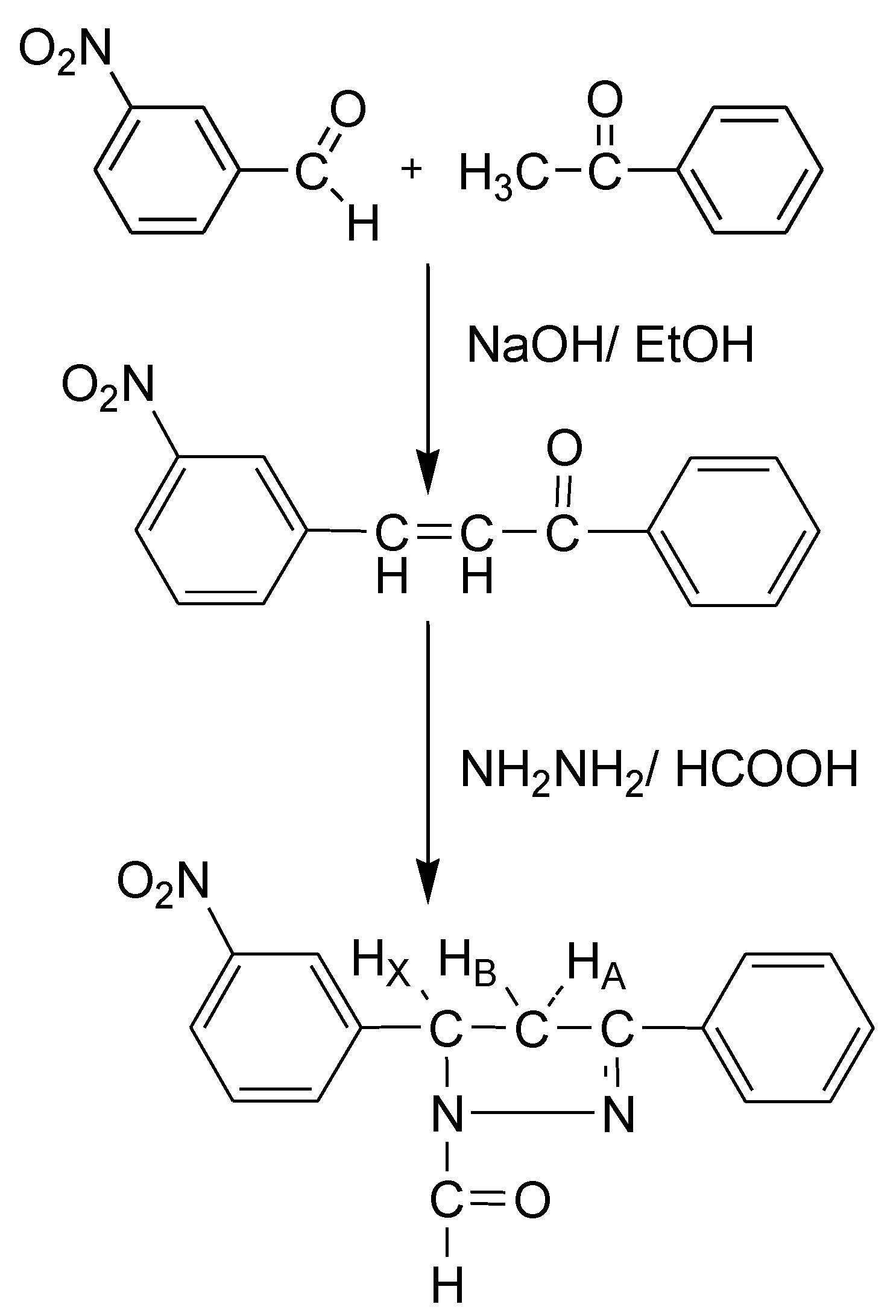
2. Results and Discussion
3. Experimental
3.1. Preparation of chalcone
3.2. Synthesis of 5-(3-nitrophenyl)-3-phenyl-4,5-dihydro-1H-pyrazole-1-carbaldehyde
4. Conclusions
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Wang, P.; Onozawa-Kamatsuzaki, N.; Himeda, Y.; Sugihara, H.; Arakawa, H.; Kasuga, K. 3-(2-Pyridyl)-2-pyrazoline derivatives: Novel fluorescent probes for Zn2+ ion. Tetrahedron Lett. 2001, 42, 9199–9201. [Google Scholar] [CrossRef]
- De Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- Elguero, J. Comprehensive Heterocyclic Chemistry; Pergamon Press: Oxford, UK, 1996; pp. 31–75. [Google Scholar]
- Catalan, J.; Fabero, F.; Claramunt, R.M.; Maria, M.D.S.; Foces-Foces, M.C.; Cano, F.H.; Martinez-Ripoll, M.; Elguero, J.; Sastre, R. New ultraviolet stabilizers: 3- and 5-(2´-hydroxyphenyl)pyrazoles. J. Am. Chem. Soc. 1992, 114, 5039–5048. [Google Scholar] [CrossRef]
- Lavai, A.; Silva, A.M.S.; Cavaleiro, J.A.S.; Alkorta, I.; Elguero, J.; Jeko, J. Synthesis of pyrazoles by treatment of 3-benzylchromones, 3-benzylflavones and their 4-thio analogues with hydrazine. Eur. J. Org. Chem. 2006, 2,825–2,832. [Google Scholar] [CrossRef]
- Khode, S.; Maddi, V.; Aragade, P.; Palkar, M.; Ronad, P.K.; Mamledesai, S.; Thippeswamy, A.H.M.; Satyanarayan, D. Synthesis and pharmacological evaluation of a novel series of 5-(substituted)aryl-3-(3-coumarinyl)-1-phenyl-2-pyrazolines as novel anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2009, 44, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- McGreer, D.E.; Morris, P.; Carmichael, G. Pyrazolines: Part III. The preparation and pyrolysis of 4,5-dimethyl-3-carbomethoxy-Δ2-pyrazoline and 3,5-dimethyl-3-carbomethoxy-Δ1-pyrazoline. Can. J. Chem. 1963, 41, 726. [Google Scholar] [CrossRef]
- Fieser, M.; Fieser, L.F. Reagents for Organic Synthesis; Wiley- Interscience: New York, NY, USA, 1969; p. 211. [Google Scholar]
- Freeman, J.P. A synthesis of cyclopropyl acetates1,2. J. Org. Chem. 1964, 29, 1379. [Google Scholar] [CrossRef]
- Nakamichi, N.; Kaeashita, Y.; Hayashi, M. Oxidative aromatization of 1,3,5-trisub-stitutedpyrazolines and Hantzsch 1,4-dihydropyridines by Pd/C in acetic acid. Org. Lett. 2002, 4, 3955–3957. [Google Scholar] [CrossRef] [PubMed]
- Bhat, B.A.; Dhar, K.; Puri, S.C.; Saxena, A.K.; Shanmugavel, M.; Qazi, G.N. Synthesis and biological evaluation of chalcones and their derived pyrazoles as potential cytotoxic agents. Bioorg. Med. Chem. Lett. 2005, 15, 3177–3180. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, W.; Michl, G.; Belaj, F.; Brun, R.; Saf, R.; Weis, R. One-pot syntheses of 2-pyrazoline derivatives. Tetrahedron 2003, 59, 2811–2819. [Google Scholar] [CrossRef]
- Butcher, R.J.; Jasinski, J.P.; Prasad, D.J.; Narayana, B.; Yathirajan, H.S. 3-(4-Methylphenyl)-5-[4-(methylthio)phenyl]-4,5-dihydro-1H-pyrazole-1-carbaldehyde. Acta Cryst. 2007, E63, o4,005–o4,006. [Google Scholar] [CrossRef]
- Kalirajan, R.; Sivakumar, S.U.; Jubie, S.; Gowramma, B.; Suresh, B. Synthesis and biological evaluation of some heterocyclic derivatives of Chalcones. Int. J. ChemTech. Res. 2009, 1, 27–34. [Google Scholar]
- Jain, M.S.; Chourasia, O.P.; Rao, J.T. Synthetic and antimicrobial studies of some new chalcones of 3-bromo-4-(p-tolyl sulphonamido) acetophenone. E-j. chem. 2004, 1, 178–183. [Google Scholar] [CrossRef]
- Kohle, E.P.; Chadwell, H.M. Benzalacetophenone. Org. Synth. 1922, 2, 1. [Google Scholar]
- Borovik, V.P.; Shkurko, O.P. Synthesis of isomeric bis(aminophenyl)pyrimidines from nitrochalcones. Russ. J. Appl. Chem. 2008, 81, 254–258. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Singh, P.; Negi, J.S.; Pant, G.J.n.; Rawat, M.S.M. 5-(3-Nitrophenyl)-3-phenyl-4,5-dihydro-1H-pyrazole-1-carbaldehyde. Molbank 2010, 2010, M650. https://doi.org/10.3390/M650
Singh P, Negi JS, Pant GJn, Rawat MSM. 5-(3-Nitrophenyl)-3-phenyl-4,5-dihydro-1H-pyrazole-1-carbaldehyde. Molbank. 2010; 2010(1):M650. https://doi.org/10.3390/M650
Chicago/Turabian StyleSingh, Pramod, Jagmohan S. Negi, Geeta Joshi nee Pant, and Mohan S.M. Rawat. 2010. "5-(3-Nitrophenyl)-3-phenyl-4,5-dihydro-1H-pyrazole-1-carbaldehyde" Molbank 2010, no. 1: M650. https://doi.org/10.3390/M650
APA StyleSingh, P., Negi, J. S., Pant, G. J. n., & Rawat, M. S. M. (2010). 5-(3-Nitrophenyl)-3-phenyl-4,5-dihydro-1H-pyrazole-1-carbaldehyde. Molbank, 2010(1), M650. https://doi.org/10.3390/M650


