1. Introduction
Quinolinyl amines are important organic compounds which possess a variety of pharmacological activities such as antimalarial, antifungal, hypotensive and antidepressant activity [1,2,3,4]. From the various conditions adopted for the synthesis of secondary amines reported in the literature, synthesis via nucleophilic substitution is still an important method [5]. As a part of our research programme on quinoline derivatives [6], we report herein the synthesis of N-[(2-chloro-6-methylquinolin-3-yl)methyl]aniline (Figure 1) by nucleophilic substitution of 2-chloro-3-(chloromethyl)-6-methylquinoline with aniline in absolute ethanol in the presence of triethylamine (TEA) as base.
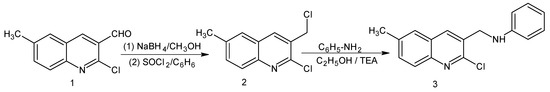
Figure 1.
Synthetic route to the title compound 3.
2. Experimental and Section
The starting material, 2-chloro-3-formyl-6-methylquinoline (1), was synthesized by the literature method [7] and it was reduced to 2-chloro-3-(hydroxymethyl)-6-methylquinoline [8] with NaBH4 in methanol.
2.1. 2-Chloro-3-(chloromethyl)-6-methylquinoline 2
To a solution of 2-chloro-3-(hydroxymethyl)-6-methylquinoline (0.01 mol, 2.07 g) in dry benzene (30 mL), SOCl2 (0.013 mol, 1.54 g) was added and the mixture was refluxed for 5 h. The solvent was removed under reduced pressure and the residue was dissolved in ether, washed with sodium bicarbonate solution (10%, 25 mL) and twice with water (25 mL), dried over sodium sulphate and concentrated in vacuo to give a residue which was crystallized from methanol. Yield, 85%; mp 140 °C, light brown colored crystals. 1H-NMR (300 MHz, CDCl3) δ: 2.53 (s, 3H, CH3), 4.87 (s, 2H, CH2), 7.55-7.57 (m, 2H, H-5 and H-7), 7.92 (d, 1H, H-8, J = 7.5 Hz), 8.17 (s, 1H, H-4). 13C-NMR (CDCl3, 75 MHz) δ: 21.48 (CH3), 43.17 (CH2), 125.91, 127.12, 127.93, 129.06, 135.76, 141.13, 147.16, 149.31. FAB-MS: 226 (M)+, 227 (M+1)+, 228 (M+2)+, 190 (M-HCl)+. Anal. Calcd for C11H9Cl2N: C, 58.43; H, 4.01; N, 6.19. Found: C, 58.30; H, 4.03; N, 6.22%.
2.2. N-[(2-Chloro-6-methylquinolin-3-yl)methyl]aniline 3
To a solution of compound 2 (0.01 mol, 2.26 g) and aniline (0.01 mol, 0.93 g) in 30 mL of absolute ethanol, 1 mL of triethylamine (TEA) was added and the mixture was refluxed for 13 h. The contents of the flask were reduced to half of its volume and left overnight. The crystalline mass obtained was filtered, washed with water, dried and recrystallized from ethanol. Yield, 78%; mp 175-176 °C, yellow crystals. 1H-NMR (300 MHz, CDCl3) δ: 2.51 (s, 3H, CH3), 4.20 (s, 1H, NH, D2O-exchangeable), 4.59 (s, 2H, CH2), 6.67 (d, 2H, H-2’ and H-6’, J = 7.3 Hz), 6.77 (t, 1H, H-4’, J = 7.1 Hz), 7.14-7.19 (m, 2H, H-3’ and H-5’), 7.53-7.55 (m, 2H, H-5 and H-7), 7.92 (d, 1H, H-8, J = 7.8 Hz), 8.09 (s, 1H, H-4). 13C-NMR (75 MHz, CDCl3) δ: 18.97 (CH3), 46.15 (CH2), 112.52, 117.03, 126.99, 127.47, 128.22, 130.02, 132.71, 136.49, 143.05, 147.17, 148.19. FAB-MS m/z: 283 (M)+, 285 (M+2)+, 190 (C11H8ClN+). Anal. Calcd for C17H15ClN2: C, 72.21; H, 5.35; N, 9.91. Found: C, 72.38; H, 5.33; N, 9.96 %.
3. Conclusions
2-Chloro-3-formyl-6-methylquinoline 1 via its reduction with solid sodium borohydride in methanol afforded 2-chloro-3-(hydroxymethyl)-6-methylquinoline 2 which on subsequent chlorination with thionyl chloride in dry benzene gave 2-chloro-3-(chloromethyl)-6-methylquinoline 3 as buff colored crystalline solid. The title compound was prepared by refluxing equimolar amounts of compound 2 and aniline in absolute ethanol in the presence of triethylamine in excellent yield. Compounds 2 and 3 were characterized by combined use of FT-IR, 1H NMR and 13C-NMR (Bruker-300 MHz) and mass spectrometry (FAB-MS). In the 1H-NMR spectrum of compound 2, the signal due to CH2Cl was observed as singlet integrating for two protons at 4.87 ppm. These methylene protons underwent slight diamagnetic shift to 4.59 ppm on substitution with the amino group of aniline in compound 3. Similarly, in the 13C-NMR spectrum of compound 2 the carbon of the CH2Cl group resonated at 43.17 ppm and underwent a slight paramagnetic shift to 46.15 ppm in compound 3 on substitution. The FAB-MS spectrum of compound 3 showed the molecular ion peak at m/z 283 and an isotopic peak at m/z 285 (M+2) and a fragment peak at m/z 190 of C11H8ClN+.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6Acknowledgement
The authors are grateful to the University Grant Commission (UGC), New Delhi, Govt. of India, for the award of UGC-JRF to one of the author SK and Central Drug Research Institute (CDRI), Lucknow, India for providing spectral data.
References
- Solomon, V.R.; Puri, S.K.; Srivastava, K.; Katti, S.B. Design and synthesis of new antimalarial agent from 4-aminoquinoline. Bioorg. Med. Chem. 2005, 13, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Gholap, A.R.; Toti, K.S.; Shirazi, F.; Kumari, R.; Bhat, M.K.; Deshpande, M.V.; Srinivasan, K.V. Synthesis and evaluation of antifungal properties of a series of the novel 2-amino-5-oxo-4-phenyl-5,6,7,8-tetrahydroquinoline-3-carbonitrile and its analogues. Bioorg. Med. Chem. 2007, 15, 6705–6715. [Google Scholar] [CrossRef] [PubMed]
- Sathi, G.; Gujrati, V.R.; Sharma, M.; Nath, C.; Bhargava, K.P.; Shanker, K. New quinolines as potential CNS agents. Arch. Pharm. 1983, 316, 767–772. [Google Scholar] [CrossRef]
- McCall, J.M.; TenBrink, R.E.; Kamdar, B.V.; Skaletzky, L.L.; Perricone, S.C.; Piper, R.C.; Delehanty, P.J. 7-(Trifluoromethyl)-4-aminoquinoline hypotensives: Novel peripheral sympatholytics. J. Med. Chem. 1986, 29, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, R.N.; Yoon, C.H.; Jung, K.W. Synthesis of secondary amines. Tetrahedron 2001, 57, 7785–7811. [Google Scholar] [CrossRef]
- Bawa, S.; Kumar, S. Synthesis of Schiff’s bases of 8-methyltetrazolo[1,5-a]quinoline as potential anti-inflammatory and antimicrobial agents. Indian J. Chem. 2009, 48B, 142–145. [Google Scholar]
- Meth-Cohn, O.; Narine, B.; Tarnowski, B. A versatile new synthesis of quinoline and related fused pyridines. Part-5. The synthesis of 2-chloroquinoline-3-carboxaldehyde. J. Chem. Soc. Perkin Trans I. 1981, 1520–1530. [Google Scholar] [CrossRef]
- Pokalwar, R.U.; Hangarge, R.V.; Kategaonkar, A.H.; Shingare, M.S. Simple and efficient synthesis of new O,O-diethyl phosphorothioates. Russ. J. Org. Chem. 2009, 45, 430–433. [Google Scholar] [CrossRef]
- Sample Availability: Sample of compound 3 is available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).