Recentely, Rubat et al. [1] synthesized a series of products by alkylation of pyridazines, the authors showed that these products are good analgesics and have a low toxicity. In our ongoing reseach program, we have synthesized compound (II); it will be subjected to further pharmacological investigations, especially tests of its anticancer activity.
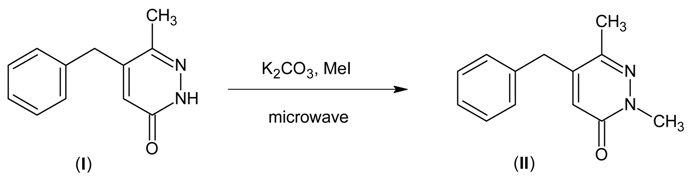
The product (II) was prepared from 5-benzyl-6-methylpyridazin-3(2H)-one (I) by solid-liquid PTC conditions without solvent [2]. To pyridazinone (I) (1.2 g, 5 mmol) were added potassium carbonate (0.692 g, 5 mmol), TBAB (0.3 g, 1 mmol) and methyl iodide (0.73 g, 5 mmol). The mixture was placed in a pyrex tube which was then introduced into a Maxidigest MX 350 Prolabo microwave monomode reactor, fitted with a rotational system. At the end of the irradiation time (10 min, 90 W irradiation power), the mixture was cooled to ambient temperature. The precipitate formed was filtered and washed with water, yield: 96% of (II).
Melting point: 89-93°C
IR (KBr): 1663 (CO), 1591 (C=N), 1430, 1495 (C=C).
1H NMR (300.14 MHz, CDCl3): δ (ppm) : 2.20 (s, 3H, CH3), 3.72 (s, 3H, CH3), 3.81 (s, 2H, CH2), 6.53 (s, 1H, H-4), 7.25 (m, 5H, aromatic protons).
13C NMR (75.48 MHz, CDCl3): δ (ppm) 19.12 (CH3), 35.85 (CH2), 39.67 (NCH3), 127.66 (CHaromatic), 127.87 (CHaromatic), 129.32 (2 CHaromatic), 129.51 (2 CHaromatic), 135.66, 145.25, 146.52, 160.63 (C=O).
Anal. Calcd for C13H14N2O: %C: 72.89; %H: 6.54;; %N: 13.08. Found: %C: 72.47; %H: 6.43; %N: 12.72.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).