Determination of the Absolute Configurations of (+)-N-((3S)-3- {[(4-methylphenyl)sulfonyl]amino}-1-oxaspiro[4.5]deca-6,9- dien-2,8-dion-7-yl) Acetamide and Benzamide
Abstract
:1. Discussion
2. Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6Acknowledgement
References and Notes:
- Plourde, G.L.; Spaetzel, R.R.; Kwasnitza, J.S.; Scully, T.W. Diastereoselective Spiroannulation of Phenolic Substrates: Advances Towards the Asymmetric Formation of the Manumycin m-C7N Core Skeleton. Molecules 2007, 12, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Plourde, G.L. Studies Towards the Diastereoselective Spiroannulation of Phenolic Derivatives. Tetrahedron Letters 2002, 43, 3597–3599. [Google Scholar] [CrossRef]
- Plourde, G.L. (±)-1-(4-Hydroxy-3-methoxyphenyl)-3-butanol. Molbank 2003, M315. [Google Scholar] [CrossRef]
- Plourde, G.L. (±)-7-Methoxy-2-methyl-1-oxaspiro[4,5]deca-6,9-diene-8-one. Molbank 2003, M316. [Google Scholar] [CrossRef]
- Plourde, G.L. 1-(4-Hydroxy-3-methoxyphenyl)-4-methyl-3-pentanone. Molbank 2003, M317. [Google Scholar] [CrossRef]
- Plourde, G.L. (±)-1-(4-Hydroxy-3-methoxyphenyl)-4-methyl-3-pentanol. Molbank 2003, M318. [Google Scholar] [CrossRef]
- Plourde, G.L. (±)-7-Methoxy-2-ipropyl-1-oxaspiro[4,5]deca-6,9-diene-8-one. Molbank 2003, M319. [Google Scholar] [CrossRef]
- Plourde, G.L. 1-(4-Hydroxy-3-methoxyphenyl)-4,4-dimethyl-3-pentanone. Mobank 2003, M320. [Google Scholar] [CrossRef]
- Plourde, G.L. (±)-1-(4-Hydroxy-3-methoxyphenyl)-4,4-dimethyl-3-pentanol. Molbank 2003, M321. [Google Scholar] [CrossRef]
- Plourde, G.L. (±)-2-tButyl-7-methoxy-1-oxaspiro[4,5]deca-6,9-diene-8-one. Mobank 2003, M322. [Google Scholar] [CrossRef]
- Plourde, G.L.; English, N.J. Diastereoselective Spiroannulation of Phenolic Substrates: Synthesis of (±)-2-tert-Butyl-6-methoxy-1-oxaspiro[4,5]deca-6,9-diene-8-one. Molecules 2005, 10, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- The configuration of the chiral centre at carbon 3 in (+)-1 and (+)-2 should be the same as the original chiral centre in (S)-3-nitrotyrosine under the reactions conditions used in the synthesis reported [(a) 1) TsCl, THF, 1M NaOH 2) 1M KOH, EtOH, 80-85 oC; (b) H2, 10% Pd/C, THF; (c) CH3COCl or PhCOCl, THF, rt; (d) PIFA, acetone, 0oC] (reference 1). Therefore, it is assumed that this centre remained in the (S)-configuration.
- Eliel, E.L.; Wilen, S.H. Stereochemistry of Organic Compounds; John Wiley & Sons, Inc.: New York, 1994; pp. 30–31. [Google Scholar]
- Silverstein, R.M.; Webster, F.X. Spectrometric Identification of Organic Compounds; 6th Edition; John Wiley & Sons, Inc.: New York, 1998; pp. 189–191. [Google Scholar]
- The distance between H3 and H10/H6 is estimated to be between 2.6Ǻ and 5Ǻ. The actual distance between these protons in a solution will depend on molecular movement and steric factors found in the molecule. This distance has not been calculated.
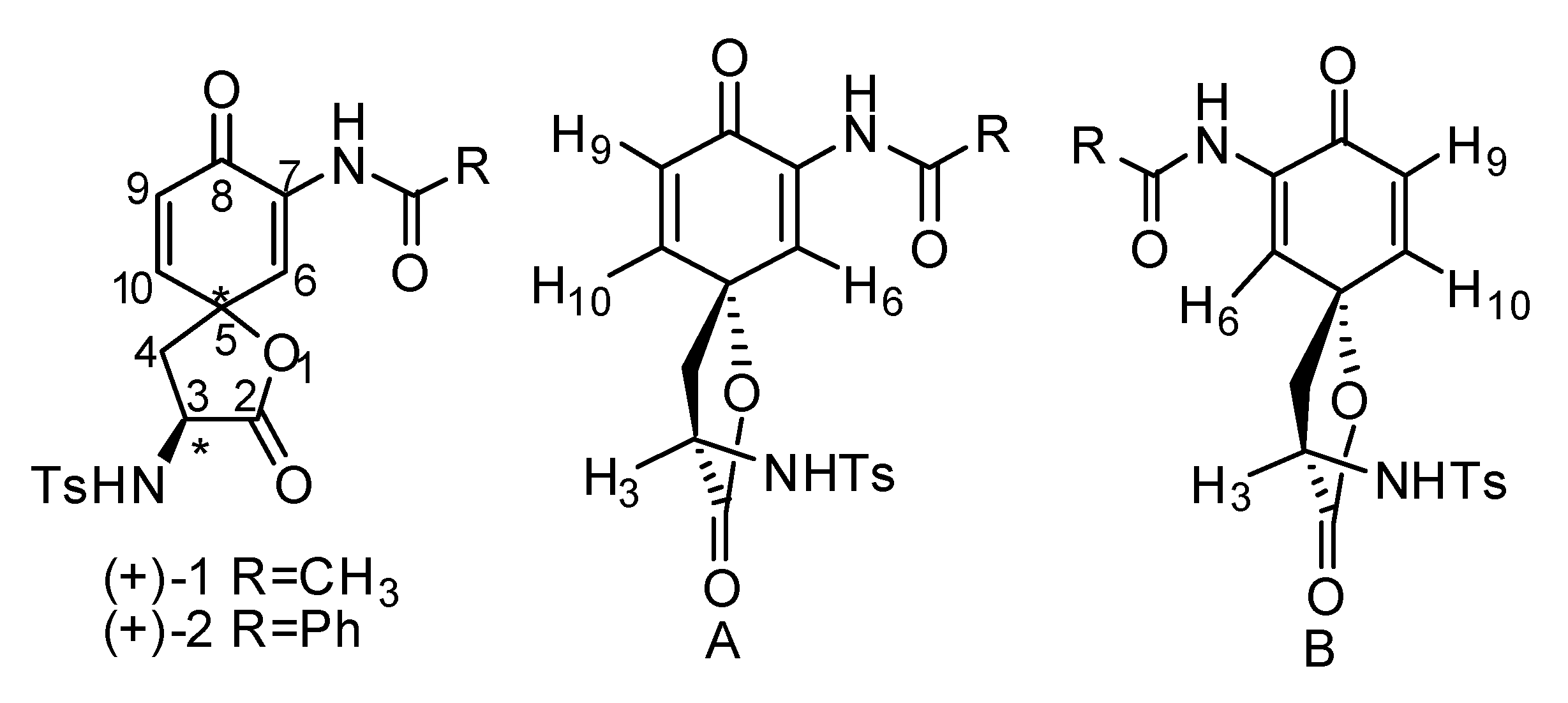
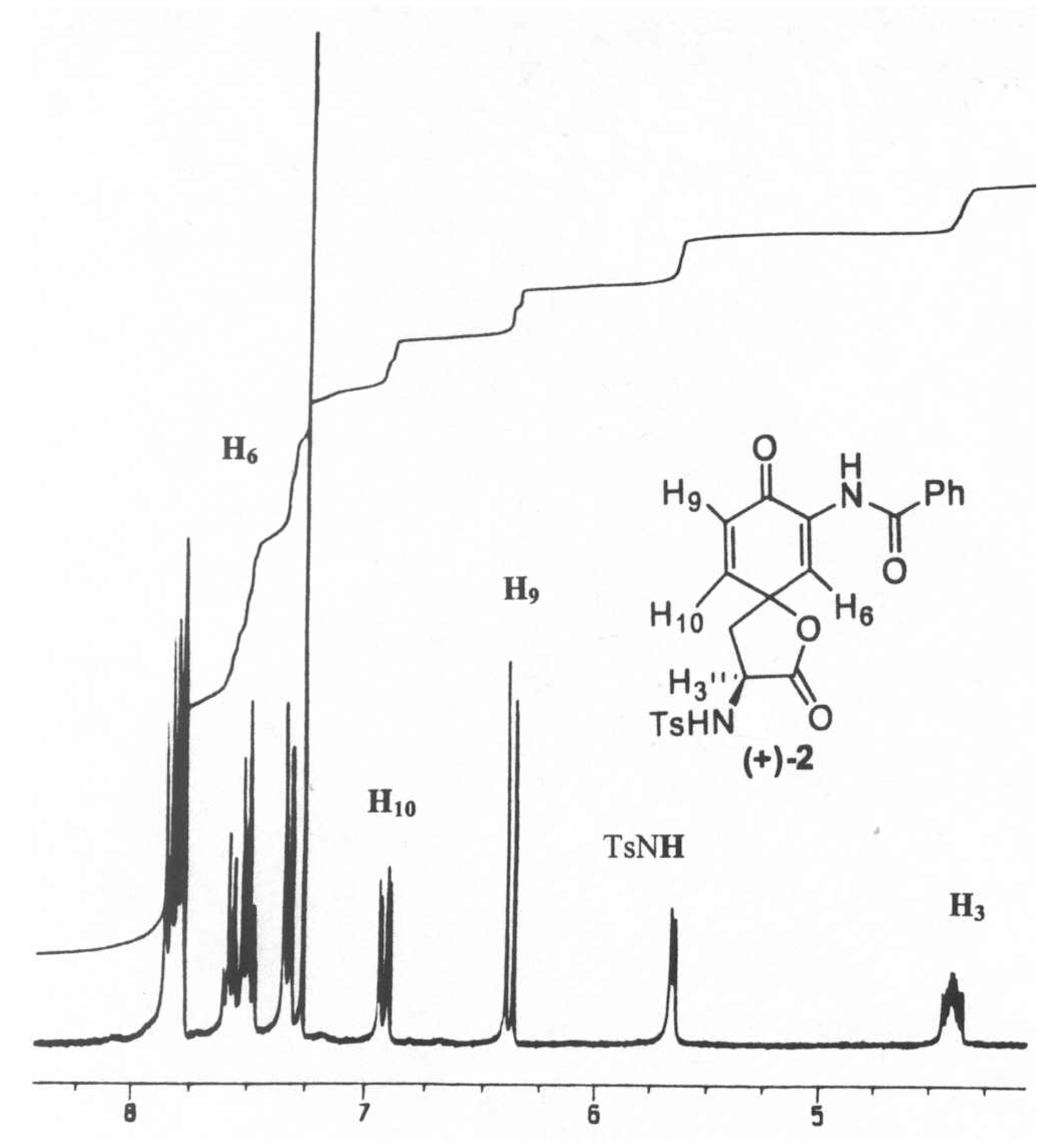
| Compound | Irradiation | Integration | |||
|---|---|---|---|---|---|
| H10 | H9 | H6 | H3 | ||
| H10 (7.06ppm) | 1.00 | 0.05 | n/a | 0.06 | |
| (+)-1 | H6 (7.52ppm) | n/a | n/a | 1.00 | n/a |
| H3 (4.54ppm) | 0.07 | n/a | n/a | 1.00 | |
| H10 (6.92ppm) | 1.00 | 0.04 | n/a | 0.05 | |
| (+)-2 | H6 (7.54ppm) | n/a | n/a | 1.00 | n/a |
| H3 (4.40ppm) | 0.04 | n/a | n/a | 1.00 | |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Plourde, G.L.; Susag, L.M.; Dick, D.G. Determination of the Absolute Configurations of (+)-N-((3S)-3- {[(4-methylphenyl)sulfonyl]amino}-1-oxaspiro[4.5]deca-6,9- dien-2,8-dion-7-yl) Acetamide and Benzamide. Molbank 2008, 2008, M579. https://doi.org/10.3390/M579
Plourde GL, Susag LM, Dick DG. Determination of the Absolute Configurations of (+)-N-((3S)-3- {[(4-methylphenyl)sulfonyl]amino}-1-oxaspiro[4.5]deca-6,9- dien-2,8-dion-7-yl) Acetamide and Benzamide. Molbank. 2008; 2008(3):M579. https://doi.org/10.3390/M579
Chicago/Turabian StylePlourde, Guy L., Lyndia M. Susag, and David G. Dick. 2008. "Determination of the Absolute Configurations of (+)-N-((3S)-3- {[(4-methylphenyl)sulfonyl]amino}-1-oxaspiro[4.5]deca-6,9- dien-2,8-dion-7-yl) Acetamide and Benzamide" Molbank 2008, no. 3: M579. https://doi.org/10.3390/M579
APA StylePlourde, G. L., Susag, L. M., & Dick, D. G. (2008). Determination of the Absolute Configurations of (+)-N-((3S)-3- {[(4-methylphenyl)sulfonyl]amino}-1-oxaspiro[4.5]deca-6,9- dien-2,8-dion-7-yl) Acetamide and Benzamide. Molbank, 2008(3), M579. https://doi.org/10.3390/M579




