Abstract
Methyl (2E)-3-methoxyacrylate and excess methylhydrazine yield crude 1-methyl-2-pyrazolin-5-one which is reacted with cyclohexanone to obtain the title compound in 86% yield. Detailed spectroscopic data (1H NMR, 13C NMR, 15N NMR, and MS) are presented.
1. Introduction
During our ongoing research on pyrano[2,3-c]pyrazol-4(1H)-ones [1,2,3,4,5] we were interested in the not yet commercially available methylpyrazolone 1. Although dozens of patents and journal articles deal with its synthesis, most of them include multi-step reactions or seem to be less suited for laboratory scale preparations. Thus, we chose to follow a patent protocol, which starts from alkoxyacrylic alkyl esters and alkylhydrazines [6]. Recently, we have successfully applied that procedure to yield the unsubstituted pyrazolone [7]. Unfortunately, no work up for the crude methylpyrazolone 1 is published in the original patent (only GC/MS analysis is carried out). When we tried to find a proper solvent to precipitate the desired methylpyrazolone from the oily reaction syrup, we found that ketones – such as acetone or cyclohexanone – readily react and give colourless precipitates as the analysis of such a precipitate revealed. A representative procedure with cyclohexanone is presented in the experimental part [Scheme 1].
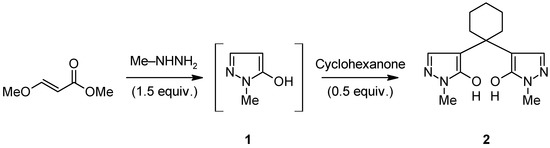
Scheme 1.
Synthesis of the title compound 2
It should be noted that according to NMR data in DMSO-d6 solution, we presume a rather unsymmetrical conformation as outlined in Scheme 2. Consistent with this suggestion, we achieved no intramolecular cyclization to the corresponding pyrano[2,3-c:6,5-c']dipyrazole upon treatment with conc. sulfuric acid or polyphosphoric acid, that would be expected if the hydroxy groups were spatially close.
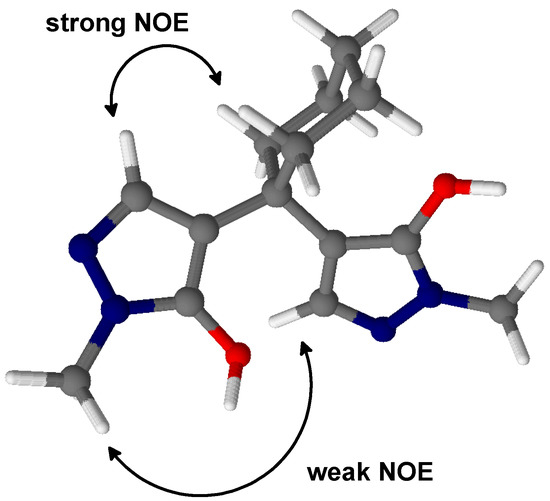
Scheme 2.
Proposed conformation of compound 2 in DMSO solution [8]
2. Experimental
Melting points were determined on a Reichert–Kofler hot-stage microscope and are uncorrected. Mass spectra were obtained on a Shimadzu QP 1000 instrument (EI, 70 eV). Elemental analysis was performed at the Microanalytical Laboratory, University of Vienna. 1H and 13C NMR spectra were recorded on a Varian UnityPlus 300 spectrometer at 28 °C (299.95 MHz for 1H, 75.43 MHz for 13C) or on a Bruker Avance 500 spectrometer at 293 K (500.13 MHz for 1H, 125.77 MHz for 13C). The centre of the solvent signal was used as an internal standard which was related to TMS with δ = 7.26 ppm (1H in CDCl3), δ = 2.49 ppm (1H in DMSO-d6), δ = 77.0 ppm (13C in CDCl3), and δ = 39.5 ppm (13C in DMSO- d6). The digital resolutions were 0.2 Hz/data point in the 1H and 0.4 Hz/data point in the 1H-coupled 13C-NMR spectra (gated decoupling). 15N NMR spectra were obtained on a Bruker Avance 500 instrument with a ‘directly’ detecting broadband observe probe and were referenced against external nitromethane (coaxial capillary).
2.1. 4,4'-(Cyclohexane-1,1-diyl)bis(1-methyl-1H-pyrazol-5-ol) (2)
CAUTION: Methylhydrazine is a potentially highly toxic compound and must be used with great care under a well-ventilated hood.
To a well stirred solution of methyl 3-methoxyacrylate (50 mmol, 5.80 g) in dry MeOH (5 mL) methylhydrazine (75 mmol, 3.46 g) was added dropwise and the mixture was stirred at room temperature for 3 h. Then the excess solvent and reagents were distilled off using a rotary evaporator to obtain the crude methylpyrazolone 1 as a yellowish honey-like mass [9]. Cyclohexanone (25 mmol, 2.45 g) was added to the residue and the mixture was refluxed for 5 min. The formed precipitate was filtered off and washed subsequently with petroleum ether and acetone to yield the pure title compound 2 (5.91 g, 86%).
Mp: 186–191 °C, colourless powder.
1H NMR (300 MHz, DMSO-d6): δ (ppm) 13.77 (br s, 2H, OH), 7.16 (s, 2H, pyrazole H-3), 3.38 (s, 6H, NMe), 2.07 (m, 4H, cyclohexane H-2,6), 1.38 (m, 6H, cyclohexane H-3,4,5).
13C NMR (75 MHz, DMSO-d6): δ (ppm) 156.0 (pyrazole C-5), 132.6 (pyrazole C-3, 1J = 183.8 Hz), 110.9 (pyrazole C-4), 33.5 (cyclohexane C-1), 33.2 (cyclohexane C-2,6), 31.6 (NMe, 1J = 140.0 Hz), 26.0 (cyclohexane C-4), 21.9 (cyclohexane C-3,5).
15N NMR (50 MHz, DMSO-d6): δ (ppm) –210.0 (N-1); N-2 was not found.
MS (m/z, %): 276 (M+, 3), 179 (42), 178 (100), 163 (50), 149 (34), 135 (33), 124 (28), 111 (48), 99 (26), 98 (53), 79 (36), 55 (45), 43 (46).
Elemental Analysis: Calculated for C14H20N4O2 (276.33): C, 60.85%; H, 7.30%; N, 20.28%. Found: C, 60.66%; H, 7.24%; N, 20.34%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6References and Notes
- Eller, G.A.; Wimmer, V.; Haring, A.W.; Holzer, W. An efficient approach to heterocyclic analogues of xanthone: a short synthesis of all possible pyrido[5,6]pyrano[2,3-c]pyrazol-4(1H)-ones. Synthesis 2006, 24, 4219. [Google Scholar] [CrossRef]
- Eller, G.A.; Haring, A.W.; Datterl, B.; Zwettler, M.; Holzer, W. Tri- and tetracyclic heteroaromatic systems: synthesis of novel benzo-, benzothieno- and thieno-fused pyrano[2,3-c]pyrazol-4(1H)-ones. Heterocycles 2007, 71, 87. [Google Scholar] [CrossRef]
- Eller, G.A.; Holzer, W. A convenient approach to heterocyclic building blocks: synthesis of novel ring systems containing a [5,6]pyrano[2,3-c]pyrazol-4(1H)-one moiety. Molecules 2007, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Eller, G.A.; Datterl, B.; Holzer, W. Pyrazolo[4',3':5,6]pyrano[2,3-b]quinoxalin-4(1H)-one: synthesis and characterization of a novel tetracyclic ring system. J. Heterocycl. Chem. 2007, 44, 1139. [Google Scholar] [CrossRef]
- Eller, G.A.; Wimmer, V.; Holzer, W. Synthesis of novel polycyclic ring systems containing two pyrano[2,3-c]pyrazol-4(1H)-one moieties. Khim. Geterotsikl. Soedin. 2007, 1251. [Google Scholar]Chem. Heterocycl. Comp. 2007, 43, 1060. [CrossRef]
- Maywald, V.; Steinmetz, A.; Rack, M.; Gotz, N.; Gotz, R.; Henkelmann, J.; Becker, H.; Aiscar Bayeto, J.J. Preparation of 1-substituted 5- or 3-hydroxypyrazoles from alkoxyacrylates and hydrazines. PCT Int. Appl. WO 0031042, 2000. [Chem. Abstr., 2000, 133, 4655]. [Google Scholar]
- Eller, G.A.; Holzer, W. A one-step synthesis of pyrazolone. Molbank 2006, M464. [Google Scholar] [CrossRef]
- ACD/3D Viewer, version 10.00. Advanced Chemistry Development, Inc.: Toronto, ON, Canada, 2006; www.acdlabs.com.
- Upon trituration and subsequent recrystallization of the oily residue with/from i.e. diethyl ether, pure methylpyrazolone 1 can be obtained as colourless crystals. Mp 108.5–111 °C. 1H NMR (500 MHz, DMSO-d6): δ (ppm) 10.90 (br s, 1H, OH), 7.09 (d, 3J(H-3,H-4) = 1.9 Hz, 1H, H-3), 5.30 (d, 3J(H-4,H-3) = 1.9 Hz, 1H, H-4), 3.47 (s, 3H, NMe). 13C NMR (125 MHz, DMSO-d6): δ (ppm) 152.6 (C-5, 2J(C-5,H-4) = 5.7 Hz, 3J(C-5,H-3) = 10.5 Hz, 3J(C-5,NMe) = 1.9 Hz), 137.1 (C-3, 1J = 182.7 Hz, 2J(C-4,H-3) = 5.1 Hz), 86.1 (C-4, 1J = 176.1 Hz), 32.9 (NMe, 1J = 139.3 Hz). 15N NMR (50 MHz, DMSO-d6): δ (ppm) −201.9 (N-1); N-2 was not found.
- Sample Availability: Compounds 1 and 2 are available from MDPI.
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).