Schiff bases from 2-hydroxy-1-naphthaldehyde have often been used as chelating ligands in the field coordination chemistry [1]. The Schiff base compounds can be classified by their photochromic and thermochromic characteristics [2].
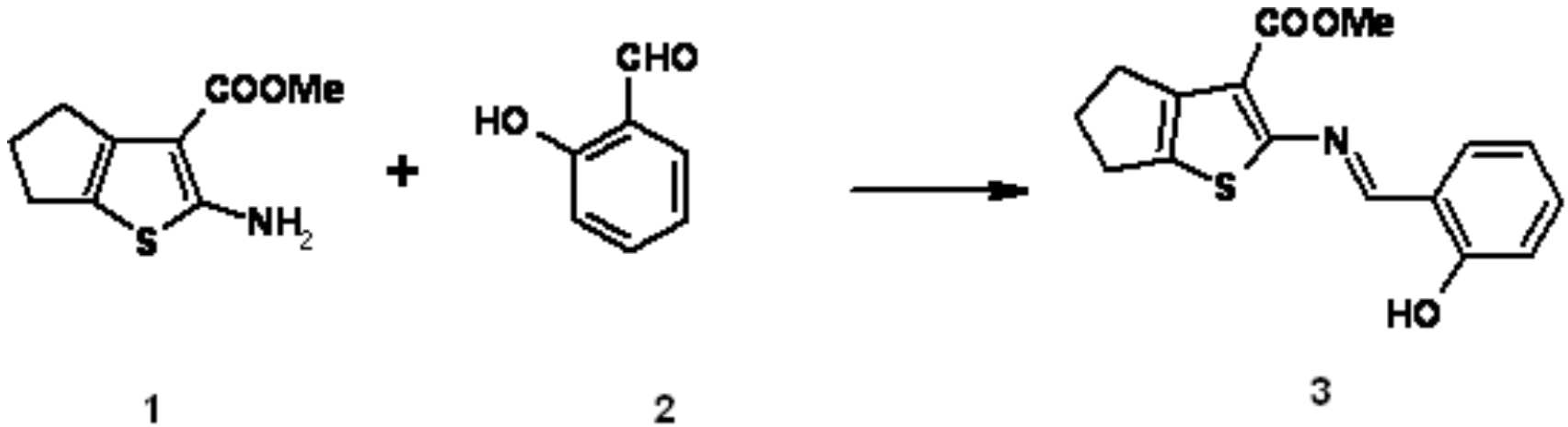
A solution of 2-Amino-5,6-dihydro-4H-cyclopenta[b]thiophene-3-carboxylic acid methyl ester 1 (2.5g, 0.0125mol) and 2-hydroxynaphthaldehyde 2 (1.53g, 0.0125 mol) in absolute ethanol (50 mL) was heated under reflux for 3 hrs. Cooling the mixture, filtering the precipitate and recrystallization from ethanol gave the Schiff base 3 as brown crystals (3.76g, 99%).
Melting Point: 135-137°C. (EtOH).
IR (KBr; cm-1); 1706.1 (C=O), 1602.1 (C=N), 1445.1 (C=C), 1209.3 (C-O) and 1033 (C-N).
¹H NMR (400 MHz;CDCl3) δ; 12.67 (s, 1H, OH), 9.19 (s, 1H, CH olefinic), 7.58 (d, 1H, CH aromatic), 7.27 (dd, 1H, CH aromatic), 6.92 (d, 1H, CH aromatic), 6.84 (dd, 1H, CH aromatic), 3.88 (s, 3H, COOMe), 3.05-3.04 (t, 2H, CH2), 2.93-2.60 (t, 2H, CH2), and 2.52-2.45 (m, 2H, CH2).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Garnovski, A.D.; Nivorozhkin, A.L.; Minkin, V.I. Coord. Chem. Rev. 1993, 126, 1.
- Hadjoudis, E.; Vittorakis, M.; Maustakali-Mavridis, I. Tetrahedron 1987, 43, 1345.
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.