Synthesis of 3-(4-Amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propanoic acid – A Functionalized Base Derivative of the Nucleoside Antibiotic Tubercidin
Abstract
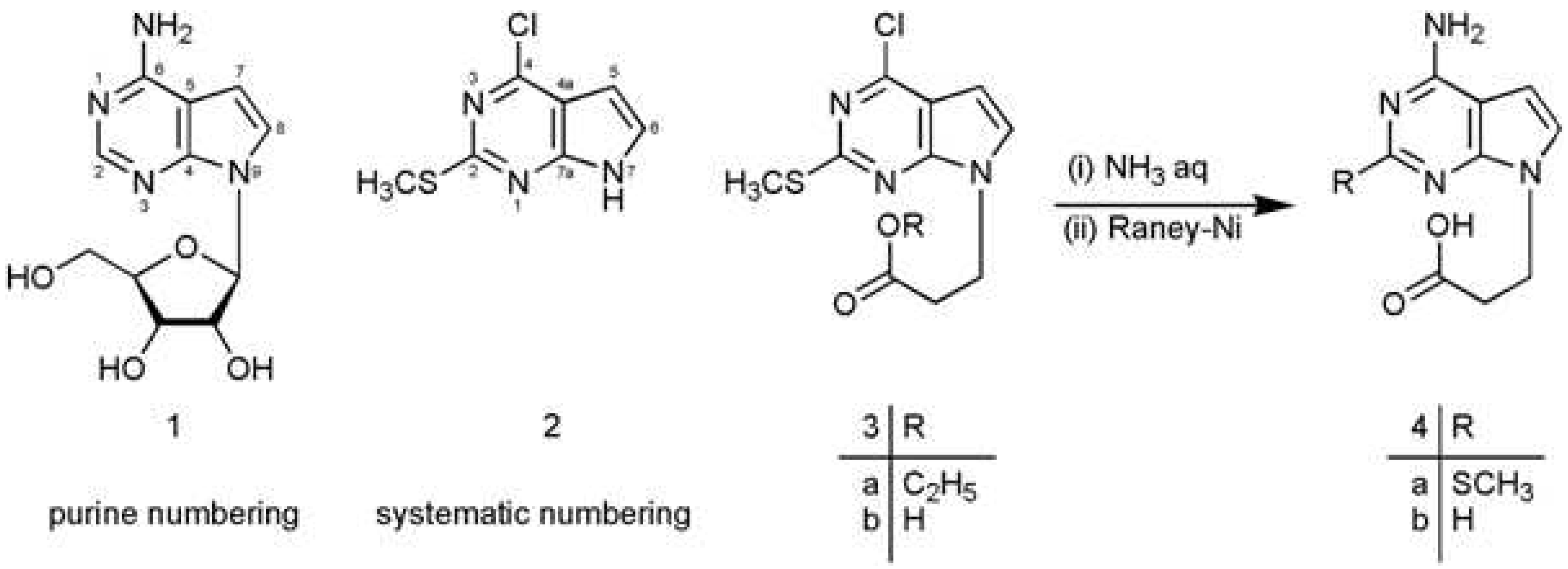
:
Experimental Procedures
General
3-[4-Chloro-2-(methylthio)-7H-pyrrolo[2,3-d]pyrimidin-7-yl]propanoic acid (3b)
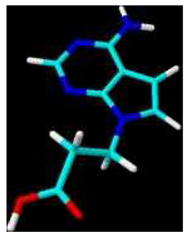
3-(4-Amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propanoic acid (4b)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6Supplementary File 7Supplementary File 8Supplementary File 9Supplementary File 10Supplementary File 11Supplementary File 12References and Notes
- Suhadolnik, R. J. Nucleosides As Biological Probes; John Wiley & Sons: New York, 1979; pp. 158–166. [Google Scholar]
- Saenger, W. Principles of Nucleic Acids Structure. In Springer Advanced Texts in Chemistry; Cantor, C. R., Ed.; Springer Verlag: New York – Berlin – Heidelberg – Tokyo, 1984; pp. 122–126, p. 243–248, and literature cited therein. [Google Scholar]
- Rosemeyer, H.; Kaiser, K.; Seela, F. Int. J. Biol. Macromol. 1987, 9, 205–210. [CrossRef]
- Ahlers, M.; Ringsdorf, H.; Rosemeyer, H.; Seela, F. Colloid Polym. Sci. 1990, 268, 132–142.
- Suhadolnik, R. J. Nucleosides As Biological Probes; John Wiley & Sons: New York, 1979; pp. 298–310. [Google Scholar]
- Uhlmann, E.; Peymann, A.; Breipohl, G.; Will, D. W. Angew. Chem. 1998, 110, 2954–2983.
- Lüpke, U. Thesis, Universität-GH Paderborn, Germany, 1979; p. 36.
- Noell, C. W.; Robins, R. K. J. Heterocyclic Chem. 1964, 1, 34–41. [CrossRef]
- Lüpke, U.; Seela, F. Chem. Ber. 1979, 112, 3432–3440.
- Compound 2 is commercially available from: Activate Scientific GmbH, Regenburg, Germany, sales@activate-scientific.com
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.
Share and Cite
Rosemeyer, H. Synthesis of 3-(4-Amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propanoic acid – A Functionalized Base Derivative of the Nucleoside Antibiotic Tubercidin. Molbank 2007, 2007, M555. https://doi.org/10.3390/M555
Rosemeyer H. Synthesis of 3-(4-Amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propanoic acid – A Functionalized Base Derivative of the Nucleoside Antibiotic Tubercidin. Molbank. 2007; 2007(4):M555. https://doi.org/10.3390/M555
Chicago/Turabian StyleRosemeyer, Helmut. 2007. "Synthesis of 3-(4-Amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propanoic acid – A Functionalized Base Derivative of the Nucleoside Antibiotic Tubercidin" Molbank 2007, no. 4: M555. https://doi.org/10.3390/M555
APA StyleRosemeyer, H. (2007). Synthesis of 3-(4-Amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propanoic acid – A Functionalized Base Derivative of the Nucleoside Antibiotic Tubercidin. Molbank, 2007(4), M555. https://doi.org/10.3390/M555



