Abstract
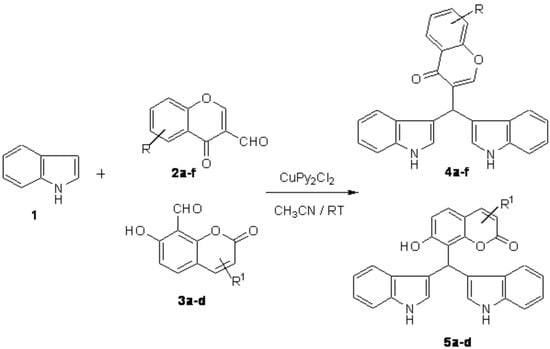
Copper dipyridine dichloride is used as an efficient catalyst for the electrophilic substitution reaction of indoles with aromatic aldehydes in acetonitrile to afford the corresponding bis (indolyl)methanes in excellent yields at room temperature.
Introduction
Indoles and their derivatives are used as antibiotics in the field of pharmaceuticals [1]. For example, Bisindolylalkanes and their derivatives are found in bioactive metabolites of terrestrial and marine origin [2]. Therefore, there is a great deal of interest in the synthesis of this class of compounds. Among the many methods, the reaction of indoles with aromatic or aliphatic aldehydes and ketones in the presence of Lewis acids, Bronsted acids or montmorillonite clay K-10, Phosphoric Acid on Silica Gel have been widely studied [3,4,5,6,7,8,9]. More recently, the use of other catalysts such as InCl3, PPh3, HClO4, LiClO4, In(OTf)3, Zeolite-HY, KHSO4 and so on [10] in acetonitrile was found to form bis(indolyl) methanes. However, many Lewis acids are deactivated or sometimes decomposed by nitrogen containing reactants. These problems can be somewhat circumvented by using expensive lithium perchlorate. However it requires longer reaction times for nitro-substituted aromatic aldehydes, giving the corresponding bis (indolyl) methanes in modereate yields. We now report here the synthesis of bis(indolyl)methanes by condensation of indoles with various aldehyde compounds using dipyridine copper chloride [11] in acetonitrile as an efficient catalyst due to the presence of two pyridine rings increases the electron deficiency on the nitrogen so it is efficiently act as a Lewis acid [12].
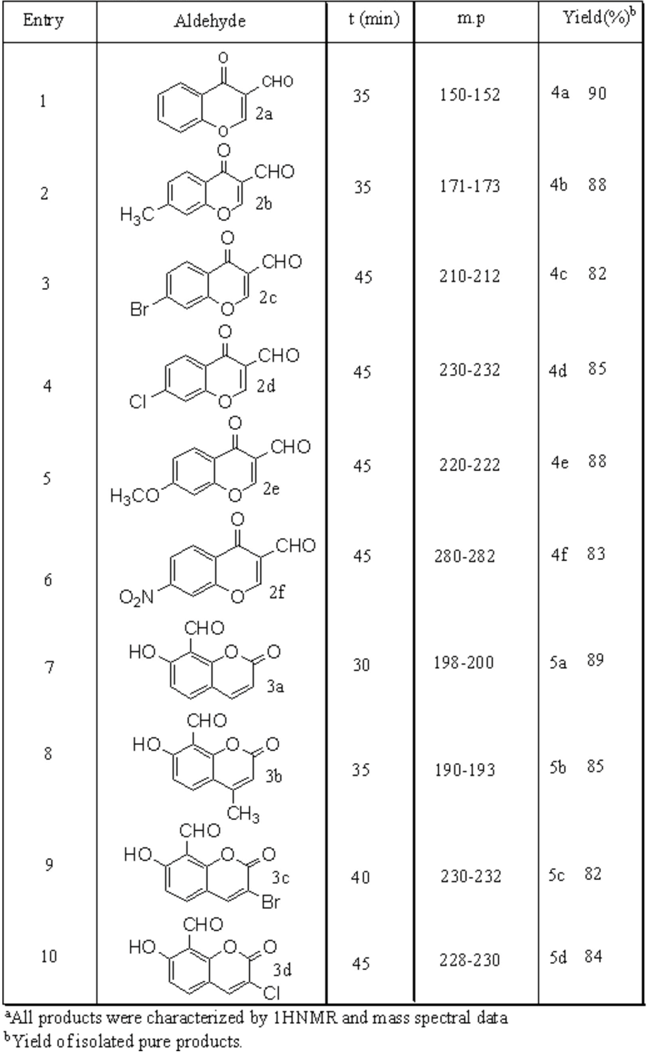
When indole (1) (10.0 mmol) was treated with various aldehydes (2 and 3) (5 mmol) in the presence of a catalytic amount of CuPy2Cl2 (10 mol%) in acetonitrile, the electrophilic substitution reactions of indoles with aldehydes proceeded smoothly at room temperature to form Bis(indolyl)methanes in almost quantitative yields. The results are summarized in Table 1.

Table 1.
CuPy2Cl2 catalyzed synthesis of Bis(Indolyl)methanesa
General Procedure
A mixture of indole (10 mmol), aldehyde (5 mmol) and copper dipyridine dichloride (10% w/w of aldehydes) in CH3CN (30 mL) was stirred at room temperature for the appropriate time. After complete conversion, as indicated by TLC, the reaction mixture was diluted with water (20 mL), and extracted with ethyl acetate (2x20 mL). The combined organic layer was dried over Na2SO4. Concentrated under reduced pressure and purified by column chromatography (ethyl acetate: hexane=1:4) to afford the pure product.
4a: 1H NMR (DMSO-d6): δ 9.31 (br s, 2H, NH), 8.01 (s, 1H, CH), 7.65 (d, 1H,Ar-H), 7.18-7.33 (m, 10H, Ar-H), 7.01 (d 1H,Ar-H ), 6.60 (d, 2H, CH), 5.25 (s, 1H), Mass: m/z (%): 390 (M+), Calcd. C, 79.98, H, 4.65, N, 7.17%. Found. C, 79.95, H, 4.63, N, 7.19%.
4b: 1H NMR (DMSO-d6): δ 9.50 (br s, 2H, NH), 8.05 (s, 1H, CH), 7.20-7.33 (m, 8H, Ar-H), 7.10 (d, 1H), 6.95 (d, 1H), 6.75 (s, 1H,), 6.45 (d, 2H,CH), 5.01(s, 1H) 2.35 (s, 3H, CH3), Mass: m/z (%): 404 (M+), Calcd. C, 80.18, H, 4.98, N, 6.93%. Found. C, 80.20, H, 4.95, N, 6.98%.
4c: 1H NMR (DMSO-d6): δ10.50 (br s, 2H, NH), 8.10 (s, 1H, CH), 7.23-7.33 (m, 8H, Ar-H), 7.01 (d, 1H), 6.90 (d, 1H), 6.75 (s, 1H,), 6.35 (d, 2H,CH), 5.25 (s, 1H), Mass: m/z (%): 463 (M+) Calcd. C, 66.54, H, 3.65, N, 5.97%. Found. C, 66.50, H, 3.69, N, 5.95%.
4d: 1H NMR (DMSO-d6): δ 10.35 (br s, 2H, NH), 7.95 (s, 1H, CH), 7.01-7.23 (m, 8H, Ar-H), 7.30 (d, 1H), 6.90 (d, 1H), 6.75 (s, 1H,), 6.40 (d, 2H,CH), 5.85 (s, 1H) Mass: m/z (%): 424 (M+), Calcd. C, 73.50, H, 4.03, N, 6.59%. Found. C, 73.54, H, 4.09, N, 6.62%.
4e: 1H NMR (DMSO-d6): δ 10.95 (br s, 2H, NH), 7.90 (s, 1H, CH), 7.22-7.31 (m, 8H, Ar-H), 7.20 (d, 1H), 6.95 (d, 1H), 6.45 (s, 1H,), 6.30 (d, 2H,CH), 5.90 (s, 1H), 3.70 (s, 3H, CH3), Mass: m/z (%): 420 (M+), Calcd. C, 77.13, H, 4.79, N, 6. 66%. Found. C, 77.10, H, 4.82, N, 6.69%.
4f: 1H NMR (DMSO-d6): δ 11.25 (br s, 2H, NH), 8.15 (s, 1H, CH), 7.32-7.45 (m, 8H, Ar-H), 7.25 (d, 1H), 6.95 (d, 1H), 6.50 (s, 1H,), 6.30 (d, 2H,CH), 5.45 (s, 1H), Mass: m/z (%): 435 (M+), Calcd C, 71.72, H, 3.94, N, 9.65%. Found. C, 71.70, H, 3.96, N, 9.67%.
5a: 1H NMR (DMSO-d6): δ 11.05 (br s, 2H, NH), 10.50 (s, 1H, OH), 8.10 (d, 1H), 7.10-7.23(m, 8H, Ar-H), 6.90 (d, 1H), 6.45 (d, 2H), 6.30 (d, 1H), 6.12 (d, 1H), 5.30 (s, 1H, CH), Mass: m/z (%): 406 (M+), Calcd. C, 76.83, H, 4.46, N, 6.89%. Found. C, 76.80, H, 4.40, N, 6.82%.
5b: 1H NMR (DMSO-d6): δ 11.20 (br s, 2H, NH), 10.45 (s, 1H, OH), 7.20-7.40 (m, 8H, Ar-H), 6.90 (d, 1H), 6.75 (d, 2H), 6.35 (d, 1H), 6.03 (s, 1H), 5.20 (s, 1H, CH), 2.60 (s, 3H, CH3), Mass: m/z (%): 420 (M+), Calcd. C, 77.13, H, 4.79, N, 6.66%. Found. C, 77.10, H, 4.73, N, 6.69%.
5c: 1H NMR (DMSO-d6): δ 11.20 (br s, 2H, NH), 10.10 (s, 1H, OH), 8.40 (s, 1H), 7.26-7.43(m, 8H, Ar-H), 6.93 (d, 1H), 6.50 (d, 2H), 6.29 (d, 1H), 5.45 (s, 1H, CH), Mass: m/z (%): 485 (M+), Calcd. C, 64.34, H, 3.53, N, 5. 77%. Found. C, 64.30, H, 3.59, N, 5.2%.
5d: 1H NMR (DMSO-d6): δ 11.50 (br s, 2H, NH), 10.45(s, 1H. OH), 8.15(s, 1H), 7.19-7.41 (m, 8H, Ar-H), 6.95 (d, 1H), 6.40 (d, 2H), 6.37 (d, 1H), 5.20 (s, 1H, CH), Mass: m/z (%): 440 (M+), Calcd. C, 70.83, H, 3.89, N, 6.35%. Found. C, 70.80, H, 3.83, N, 6.40%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6Acknowledgement
The authors greatly acknowledge the CSIR (HRDG/No 01 (2061)/06/EMR-II) for the sanction of major research project
References
- Undberg, R.J. The Chemistry of Indoles; Academic Press: New York, 1996. [Google Scholar]
- (a) Porter, J.K.; Bacon, C.W.; Robins, J.D.; Himmelsbach, D.S.; Higman, H.C. Indole alkaloids from Balansia epichloe. J. Agric .Food Chem. 1977, 25, 88–93. [Google Scholar] [CrossRef] (b) Osawa, T.; Namiki, M. Structure elucidation of streptindole, a novel genotoxic metabolite isolated from intestinal bacteria. Tetrahedron Lett. 1983, 24, 4719–4722. [Google Scholar] [CrossRef] (c) Fahy, E.; Potts, B.C.M.; Faulkner, D.J. 6-Bromotryptamine Derivatives from the Gulf of California Tunicate Didemnum candidum. J. Nat.Prod. 1991, 54, 564–569. [Google Scholar] [CrossRef] (d) Bifulco, G.; Bruno, I.; Riccio, R.; Lavayre, J.; Bourdy, G.J. Further Brominated Bis- and Tris-Indole Alkaloids from the Deep-Water New Caledonian Marine Sponge Orina sp. J. Nat. Prod. 1995, 58, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L.; Schinior, R. The Chemistry of Neuraminic Acids. I. The Ehrlich Reaction. J. Org.Chem. 1962, 27, 3696–3697. [Google Scholar] [CrossRef]
- Woland, W.; Venkiteswaren, M.; Richards, C. Cyclizative Condensations. I. 2-Methylindole with Acetone and Methyl Ethyl Ketone. J. Org. Chem. 1961, 26, 4241–4248. [Google Scholar]
- Roomi, M.; MacDonald, S. Reductive C-alkylation. II. Can. J. Chem. 1970, 48, 139–143. [Google Scholar] [CrossRef]
- Banerji, J.; Chatterjee, A.; Manna, S.; Pascard, C.; Prange, T.; Shoolery, J. Lewis Acid Induced Electrophilic Substitution of Indole. Heterocycles 1981, 15, 325–336. [Google Scholar] [CrossRef]
- Chatterjee, A.; Manna, S.; Banerji, J.; Pascard, C.; Prange, T.; Shoolery, J. Lewis-acid-induced electrophilic substitution in indoles with acetone. J. Chem. Soc., Perkin Trans. 1980, 553–555. [Google Scholar] [CrossRef]
- Gregorovich, B.; Liang, K.; Clugston, D.; Mac-Donald, S. Reductive C-alkylation. Can. J. Chem. 1968, 46, 3291–3300. [Google Scholar] [CrossRef]
- Chen, D.; Yu, L.; Wang, P.G. Lewis acid-catalyzed reactions in protic media. Lanthanide-catalyzed reactions of indoles with aldehydes or ketones. Tetrahedron Lett. 1996, 37, 4467–4470. [Google Scholar] [CrossRef]
- (a) Nagarajan, R.; Perumal, P.T. InCl3 and In(OTf)3 catalyzed reactions: synthesis of 3-acetyl indoles, bis-indolylmethane and indolylquinoline derivatives. Tetrahedron 2002, 58, 1229–1232. [Google Scholar] [CrossRef] (b) Nagarajan, R.; Perumal, P.T. Electrophilic substitution of indoles catalyzed by triphenyl phosphonium perchlorate: synthesis of 3-acetyl indoles and bis-indolylmethane derivatives. Synth. Commun. 2002, 32, 105–109. [Google Scholar] [CrossRef] (c) Bandgar, B.P.; Shaikh, K.A. Molecular iodine-catalyzed efficient and highly rapid synthesis of bis(indolyl)methanes under mild conditions. Tetrahedron Lett. 2003, 44, 1959–1961. [Google Scholar] [CrossRef] (d) Vijender Reddy, A.; Ravinder, K.; Niranjan Reddy, V.L.; Venkateswer Goud, T.; Ravikanth, V.; Venkateswarlu, V. Zeolite Catalyzed Synthesis of bis(Indolyl) Methanes. Synth. Commun. 2003, 33, 3687–3694. [Google Scholar] [CrossRef] (e) Nagarajan, R.; Permal, P.T. Chem. Lett. 2004, 33, 288–289.
- Duntitz, J. D. The crystal structures of copper dipyridine dichloride and the violet form of cobalt dipyridine dichloride. Acta Cryst. 1957, 10, 307–313. [Google Scholar] [CrossRef]
- Naveen Kumar, V.; Someshwar, P.; Narasimha Reddy, P.; Thirupathi Reddy, Y.; Rajitha, B. Copper dipyridine dichloride as a mild and efficient catalyst for the one pot condensation of biginelli reaction. J. Heterocyclic. Chem. 2005, 42, 1017–1019. [Google Scholar] [CrossRef]
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.