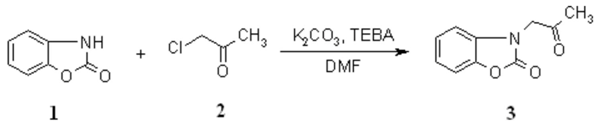
3-(2-Oxopropyl)-2(3H)-benzoxazolone
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Lozanova, Ch.; Kalcheva, V.; Simov, D. Khim. Geterosikl. Soedin. 1988, 10, 1362.
- Lozanova, Ch.; Kalcheva, V. J. Prakt. Chem. 1989, 331(6), 1007.
- Bojtscheva, Ch.; Simov, D.; Kaltscheva, V. J. Prakt. Chem. 1979, 321(2), 226.
- Zinner, H.; Randow, F. J. Prakt. Chem. 1961, 4, 144.
- Simov, D. A.; Kalcheva, V.B.; Boycheva, H.S. Compt. Rend. Acad. Bulg. Sci. 1974, 27, 1073.
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.
Share and Cite
Petrov, O.I.; Ivanova, Y.B.; Gerova, M.S.; Petrova, K.V. 3-(2-Oxopropyl)-2(3H)-benzoxazolone. Molbank 2007, 2007, M552. https://doi.org/10.3390/M552
Petrov OI, Ivanova YB, Gerova MS, Petrova KV. 3-(2-Oxopropyl)-2(3H)-benzoxazolone. Molbank. 2007; 2007(3):M552. https://doi.org/10.3390/M552
Chicago/Turabian StylePetrov, Ognyan I., Yordanka B. Ivanova, Mariana S. Gerova, and Katya V. Petrova. 2007. "3-(2-Oxopropyl)-2(3H)-benzoxazolone" Molbank 2007, no. 3: M552. https://doi.org/10.3390/M552
APA StylePetrov, O. I., Ivanova, Y. B., Gerova, M. S., & Petrova, K. V. (2007). 3-(2-Oxopropyl)-2(3H)-benzoxazolone. Molbank, 2007(3), M552. https://doi.org/10.3390/M552



