Wermuth and coll [1] synthesized a series of products by alkylation of pyridazines, Laborit [2] showed that these products are good analgesics and have a low toxicity. In continuation of this line of investigation, we have synthesized compound (I); it will be subjected to further pharmacological investigations, especially tests of its anticancer activity.
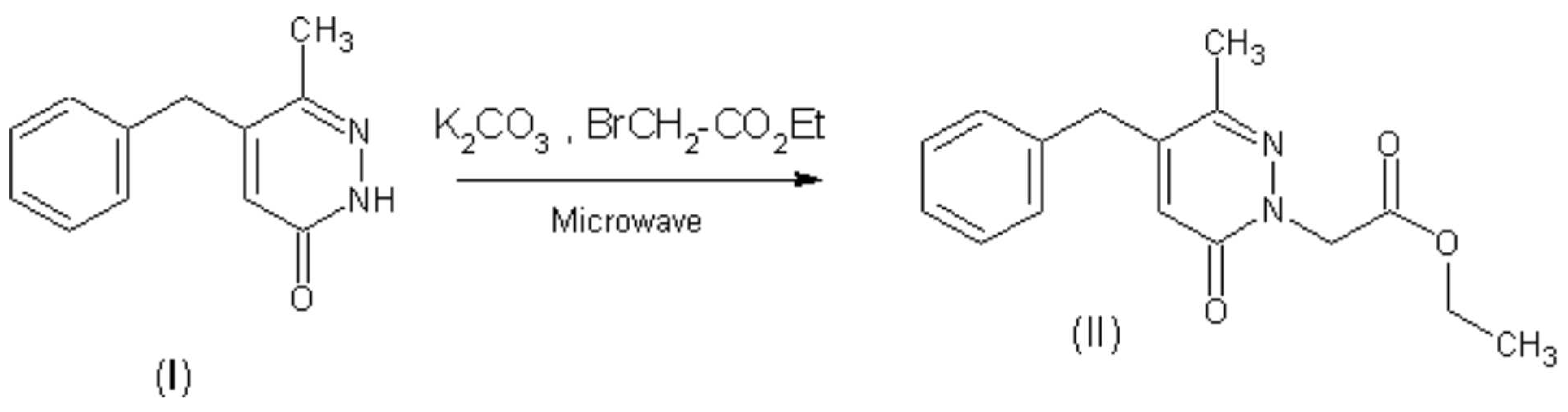
The product (II) was prepared from 5-benzyl-6-methylpyridazin-3(2H)-one (I) in situ by the solid-liquid PTC conditions without solvent[3]. To pyridazin (I) (1.2 g, 6 mmol) was added (2.75 g, 9 mmol) of potassium carbonate, (0.3 g, 1 mmol) of TBAB and (1 g, 6 mmol) of 2-ethyl bromoacetate. The mixture was placed in a pyrex tube which was then introduced into a Maxidigest MX 350 Prolabo microwave monomode reactor fitted with a rotational system. At the end of the irradiation time (10 min on 90 w as irradiation power), the mixture was cooled to ambiant temperature. After elution with ethyl acetate (30 ml) and subsequent filtration on florisil, the organic product was purified by chromatography on silicagel using CH2Cl2 as eluent, yield : 96 % of (II) solid.
Melting point: 89-93°C
IR (KBr, cm−1): 1740 (CO2Et), 1603, 1469, 1211 (C = N).
1H-NMR (300.14 MHz, CDCl3) d (ppm): 1.29 (t, J=5Hz, 3H, CH3), 2.23 (s, 3H, CH3), 3.78 (s, 2H, CH2), 4.26 (q, J = 5Hz, 2H, CH2), 6.53 (s, 1H, H4), 7.12 (d, J = 7.5Hz, 2H, aromatic protons), 7.25 (m, 3H, aromatic protons).
13C-NMR (75 MHz, CDCl3) d (ppm):19.12(CH3), 38.45 (CH2), 52.67 (CH2), 61.65 (CH2), 127.26 (CH aromatic), 127.97 (CH aromatic), 129.02 (2 CH aromatic), 129.11 (2 CH aromatic), 135.61, 145.21, 146.42, 160.38 (C3), 167.74 (C=O).
MS: m/z (%): M+= 287, 241.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References:
- Wermuth, C.G.; Leclerc, G.; Melounov, P. Chim. Ther. 1971, 2, 109.
- Laborit, H.; Weber, B.; Wermuth, C.G.; Delbarre, B.; Chekler, C.; Baron, C.; Rosen Garten, H. AGRESSOLOGIE 1965, 6, 415. [PubMed]
- (a) Kappe, C.O.; Dallinger, D. Nature Reviews Drug Discovery 2006, 5, 51. (b) De la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Chem. Soc. Rev. 2005, 34, 164. [PubMed]
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.