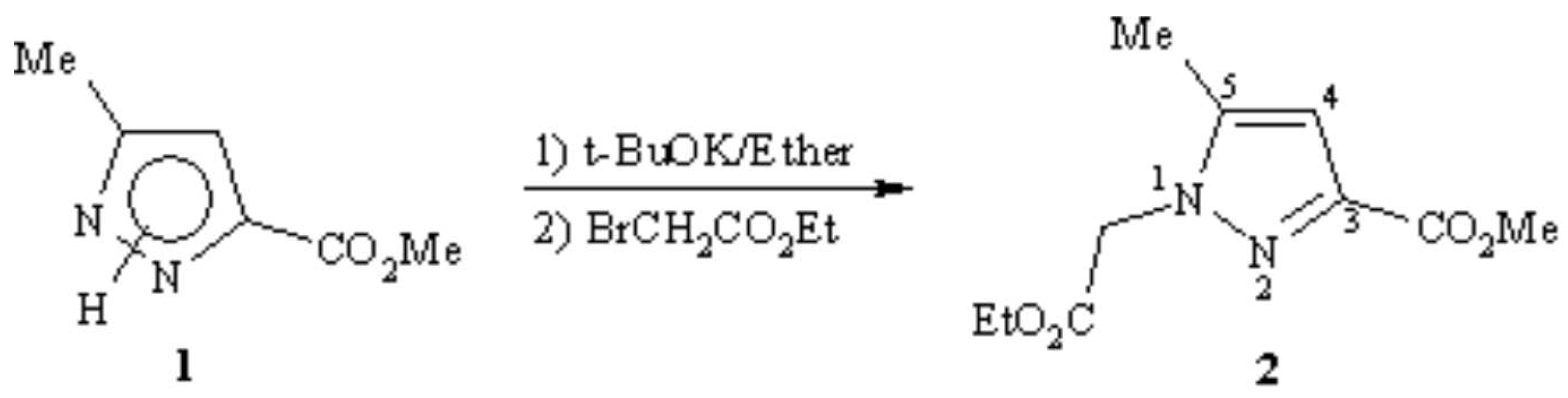
A mixture of 1 (2g; 14.3mmol) and potassium tert-butoxide (1.68g; 15mmol) in 80ml of anhydrous diethyl ether was refluxed for 75min. After cooling at 0°C, a solution of ethylbromoacetate (3.27g; 19.6mmol) in 15ml of anhydrous diethyl ether was gradually added. The reaction mixture was stirred for one night at room temperature then filtered and the solvent was eliminated under reduced pressure. The obtained residue was purified on alumina using hexane as eluant to afford a 30% yield of the new compound 2 (white solid) (0.97g; 4.3mmol). The second waited isomer has not been isolated. The product 2 was identified on the basis of its spectroscopic data. The coupling constant of the 1H NMR between the proton in position 4 and the methyl group of pyrazol ring 4J(Me-H4) was 0.71Hz. This coupling is characteristic of a 5-methyl group [1,2,3].
Melting point: 54-56°C
1H-NMR (CDCl3, 300 MHz): δ= 6.41 (s, 1H, Pz-H); 4.80 (s, 2H, N-CH2-); 4.15 (q, 2H, -CH2-CH3 , J=7.13 Hz); 3.78 (s, 3H, -O-CH3); 2.20 (s, 3H, -CH3);1.23 (t, 3H, CH2-CH3 , J=7.13 Hz).
13C-NMR (CDCl3, 75 MHz): δ= 168.04; 163.45; 144.35; 142.62; 111.61; 64.95; 54.91; 54.22; 17.00; 13.49.
IR (KBr, cm-1): 1715 and 1735 (C=O).
Elemental analysis calculated for C10H14N2O4: C 53.09, H 6.24, N 12.38. Found: C 53.42, H 6.22, N 12.07.
Mass Spectrometry (ESI): m/z =227(M+1)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References:
- Tarrago, G.; Ramdani, A.; Elguero, J.; Espada, M. J. Heterocycl. Chem. 1980, 17, 137.
- Gready, J.E.; Hatton, P.M.; Sternhell, S. J. Heterocycl. Chem. 1992, 29, 935.
- Kumar, D.; Singh, S.P.; Martinez, A.; Fruchier, A.; Elguero, J.; Martinez-Ripoll, M.; Carrio, J.S.; Virgili, A. Tetrahedron 1995, 51, 4891.
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.