Indole derivatives fall into an important class of organic compounds. These compounds are found in various natural products as fundamental nuclei and are well recognized for their wide spectrum of pharmacological and biochemical behavior. They have received the attention of biochemists because of their therapeutic and biochemical activities [1,2,3].
We report in this work the synthesis of new indoline derivatives.
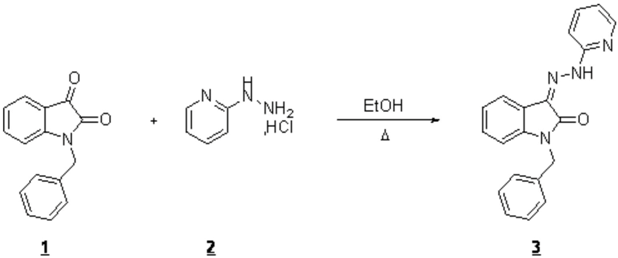

To a solution of 1 (1 g, 4.2 mmol) in 10 mL of ethanol, was added 2-hydrazinopyridine hydrochloride 2 (0.62 g, 4.2 mmol). The mixture was refluxed for 10 h. The precipitate was filtered and washed with ethanol to give compound 3, which was recrystallized from methanol.
Yield: (83%).
Melting Point: 155 °C.
MS (EI): M+ (m/z) = 328.
1H NMR (300 MHz, CDCl3): 5.05 (s, 2H, NCH2); 6.55-8.30 (m, 3H, HAr); 12.91 (s, 1H, NH).
13C NMR (300 MHz, CDCl3): 43.3 (NCH2); 108.1, 109.5, 118.3, 119.4, 122.7, 127.3, 127.7, 128.8, 138.1, 148.2 (CHAr); 121.0, 128.7, 135.6, 141.0 (Cq); 155.3 (C=Nimine); 161.7 (C=Oamide).
Elemental analysis: Calculated for C20H16N4O: C, 73.15%; H, 4.91%; N, 17.06%; Found: C, 73.01%; H, 5.05%; N, 16.89%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Verma, M.; Pandeya, S.N.; Singh, K.N.; Stables, J.P. Acta Pharm. 2004, 54, 49. [PubMed]
- Guengerich, F.P.; Sorrells, J.L.; Schmitt, S.; Krauser, J.A.; Aryal, P.; Meijer, L. J. Med. Chem. 2004, 47, 3236. [PubMed]
- Pirrung, M.C.; Pansare, S.V.; Sarma, K.D.; Keith, K.A.; Kern, E.R. J. Med. Chem. 2005, 48, 3045. [PubMed]
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.