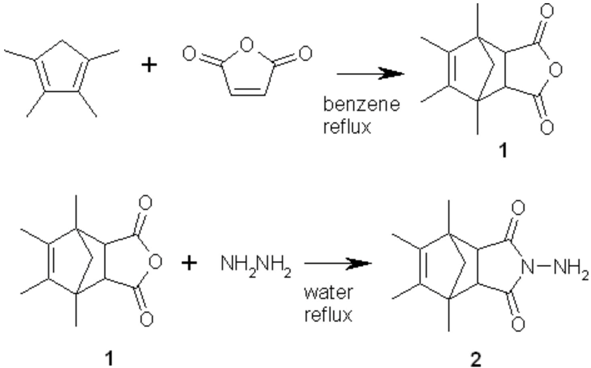
Synthesis of 4-Amino-1,7,8,9-tetramethyl-4-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6References
- Kossakowski, J.; Kuśmierczyk, J. Acta Pol. Pharm. 1999, 56, 94.
- Mironov, V.A.; Sobolev, E.V.; Elizarova, A.N. Tetrahedron 1963, 19, 1939.
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.
Share and Cite
Struga, M.; Kossakowski, J. Synthesis of 4-Amino-1,7,8,9-tetramethyl-4-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione. Molbank 2007, 2007, M534. https://doi.org/10.3390/M534
Struga M, Kossakowski J. Synthesis of 4-Amino-1,7,8,9-tetramethyl-4-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione. Molbank. 2007; 2007(2):M534. https://doi.org/10.3390/M534
Chicago/Turabian StyleStruga, Marta, and Jerzy Kossakowski. 2007. "Synthesis of 4-Amino-1,7,8,9-tetramethyl-4-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione" Molbank 2007, no. 2: M534. https://doi.org/10.3390/M534
APA StyleStruga, M., & Kossakowski, J. (2007). Synthesis of 4-Amino-1,7,8,9-tetramethyl-4-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione. Molbank, 2007(2), M534. https://doi.org/10.3390/M534



