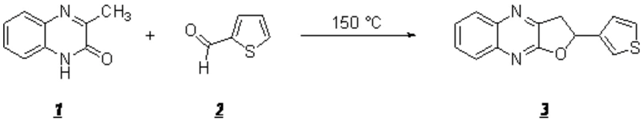
2-(3-thienyl)-2,3-dihydrofuro[2,3-b]quinoxaline
Supplementary materials
Supplementary File 1References
- Carta, A.; Paglietti, G.; Nikookar, M.E.R.; Sanna, P.; Sechi, L.; Zanetti, S. Eur. J. Med. Chem. 2002, 37, 355. [PubMed]
- Carta, A.; Loriga, M.; Zanetti, S.; Sechi, L.A. Il Farmaco 2003, 58, 1251. [PubMed]
- Fonseca, T.; Gigante, B.; Marques, M.M.; Gilchrist, T.L.; De Clercq, E. Bioorg. Med. Chem. 2004, 12, 103. [PubMed]
- Sehlstedt, U.; Aich, P.; Bergman, J.; Vallberg, H.; Nordén, B.; Gräslund, A. J. Mol. Bio. 1998, 278, 31.
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.
Share and Cite
Anothane, C.A.; Essassi, E.M. 2-(3-thienyl)-2,3-dihydrofuro[2,3-b]quinoxaline. Molbank 2007, 2007, M536. https://doi.org/10.3390/M536
Anothane CA, Essassi EM. 2-(3-thienyl)-2,3-dihydrofuro[2,3-b]quinoxaline. Molbank. 2007; 2007(2):M536. https://doi.org/10.3390/M536
Chicago/Turabian StyleAnothane, Caleb Ahoya, and El Mokhtar Essassi. 2007. "2-(3-thienyl)-2,3-dihydrofuro[2,3-b]quinoxaline" Molbank 2007, no. 2: M536. https://doi.org/10.3390/M536
APA StyleAnothane, C. A., & Essassi, E. M. (2007). 2-(3-thienyl)-2,3-dihydrofuro[2,3-b]quinoxaline. Molbank, 2007(2), M536. https://doi.org/10.3390/M536



