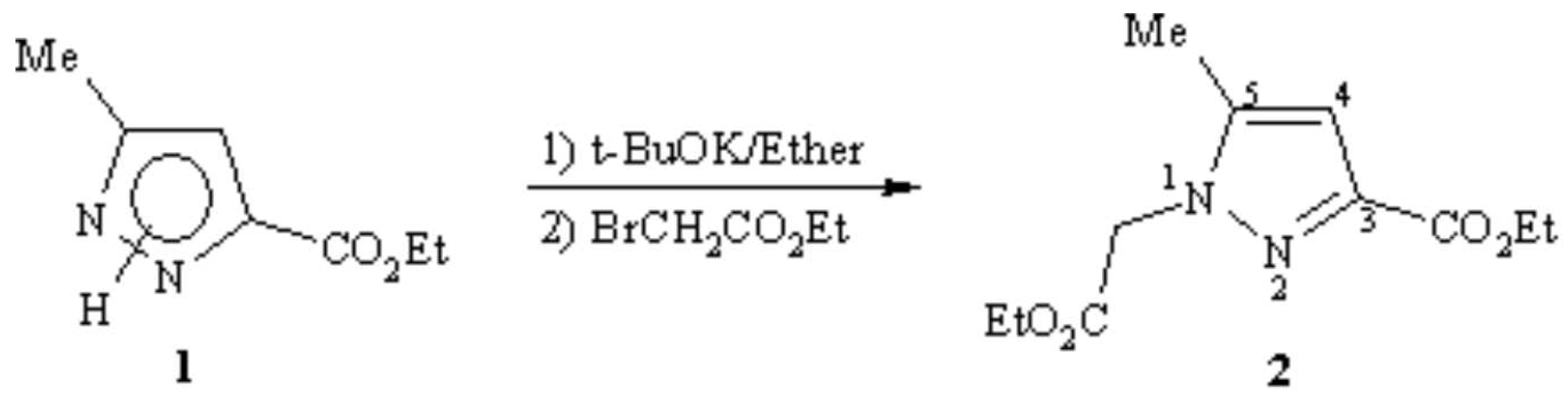
1-(2-ethoxy-2-oxoethyl)-5-methyl-1H-pyrazole-3-ethyl carboxylate
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References:
- Tarrago, G.; Ramdani, A.; Elguero, J.; Espada, M. J. Heterocycl. Chem. 1980, 17, 137.
- Gready, J.E.; Hatton, P.M.; Sternhell, S. J. Heterocycl. Chem. 1992, 29, 935.
- Kumar, D.; Singh, S.P.; Martinez, A.; Fruchier, A.; Elguero, J.; Martinez-Ripoll, M.; Carrio, J.S.; Virgili, A. Tetrahedron 1995, 51, 4891.
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.
Share and Cite
Attayibat, A.; Radi, S.; Lekchiri, Y.; Ramdani, A.; Bacquet, M. 1-(2-ethoxy-2-oxoethyl)-5-methyl-1H-pyrazole-3-ethyl carboxylate. Molbank 2007, 2007, M527. https://doi.org/10.3390/M527
Attayibat A, Radi S, Lekchiri Y, Ramdani A, Bacquet M. 1-(2-ethoxy-2-oxoethyl)-5-methyl-1H-pyrazole-3-ethyl carboxylate. Molbank. 2007; 2007(2):M527. https://doi.org/10.3390/M527
Chicago/Turabian StyleAttayibat, Ahmed, Smaail Radi, Yahya Lekchiri, Abdelkrim Ramdani, and Maryse Bacquet. 2007. "1-(2-ethoxy-2-oxoethyl)-5-methyl-1H-pyrazole-3-ethyl carboxylate" Molbank 2007, no. 2: M527. https://doi.org/10.3390/M527
APA StyleAttayibat, A., Radi, S., Lekchiri, Y., Ramdani, A., & Bacquet, M. (2007). 1-(2-ethoxy-2-oxoethyl)-5-methyl-1H-pyrazole-3-ethyl carboxylate. Molbank, 2007(2), M527. https://doi.org/10.3390/M527



