We describe in this work the synthesis of new benzodiazepine derivatives susceptible to possess various pharmacological activities.
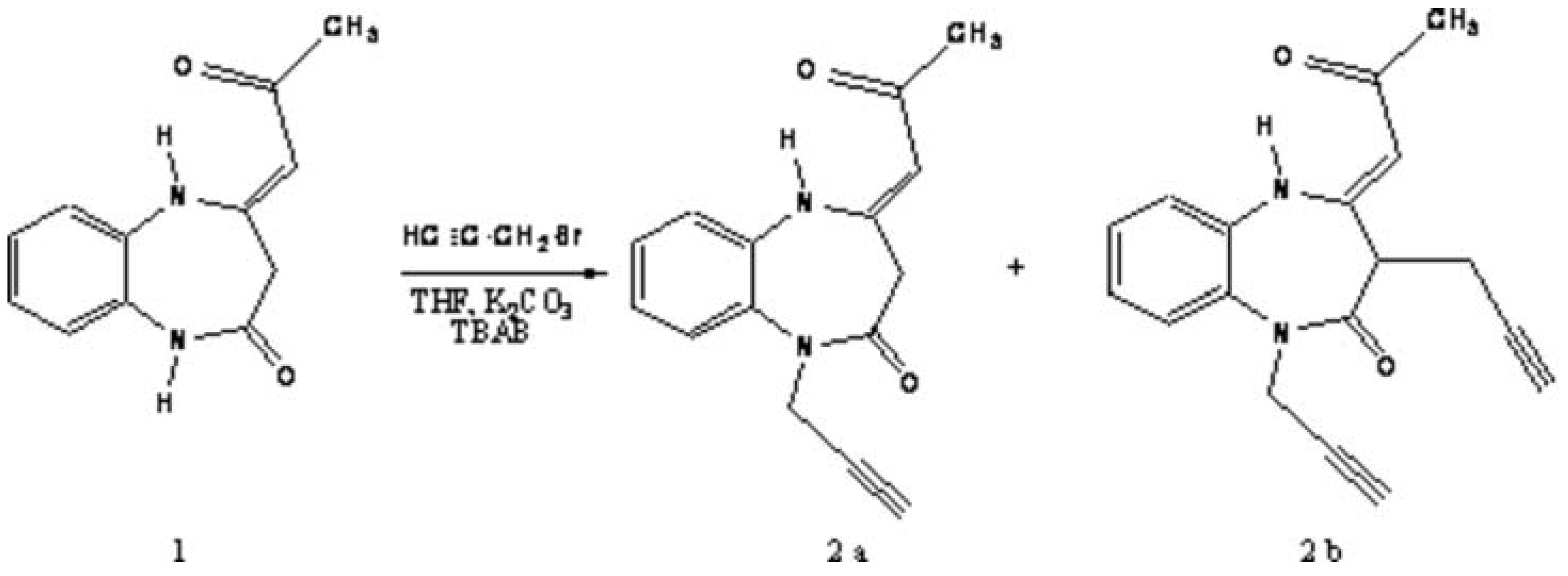
To a solution of (4Z) -2-oxopropylidene-1,5-benzodiazepin-2-one 1[1,2] (0,01 mole, 2,16 gm in tetrahydrofuran 60 mL, was added K2CO3 (0.02mole, 2,76 gm), propargylbromid (0,02 mole, 2,36 gm) and tetra n-butylammonium bromid TBAB (0,001 mole, 0,321 gm). The mixture was stirred at room temperature for 48 hours. The solution was filtered by suction filtration. The solvent was removed under reduced pressure. The residue was chromatographed on silica gel column using hexane and ethyl acetate (80/20) as eluent to afford the products 2a and 2b as white solids.
(4Z)-1-Propargyl-4-(2-oxopropylidene)-1,3,4,5-tetrahydro-2H-1,5-benzodiazepin-2-one, 2a
This compound was obtained in 50% yield;
Melting Point: 172-174 °C
1H-NMR (250 MHz, CDCl3): δ= 2.1 (s,3H,CH3),2.3(t,1H,4J=2.4 Hz, 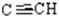 ), 3.1(HAHB, J=11.8 Hz,
), 3.1(HAHB, J=11.8 Hz, 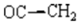 ), 4.7(HAHB, 2J=17.17 Hz, 4J=2.4 Hz,
), 4.7(HAHB, 2J=17.17 Hz, 4J=2.4 Hz, 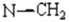 ), 5.3(s,1H,
), 5.3(s,1H, 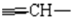 ),7.1-7.3(m, 4H, HArom)
),7.1-7.3(m, 4H, HArom)
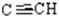 ), 3.1(HAHB, J=11.8 Hz,
), 3.1(HAHB, J=11.8 Hz, 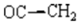 ), 4.7(HAHB, 2J=17.17 Hz, 4J=2.4 Hz,
), 4.7(HAHB, 2J=17.17 Hz, 4J=2.4 Hz, 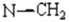 ), 5.3(s,1H,
), 5.3(s,1H, 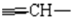 ),7.1-7.3(m, 4H, HArom)
),7.1-7.3(m, 4H, HArom)13C-NMR (62.9 MHz, CDCl3): δ= 29.4, 38.5, 41.2, 72.5, 79.0, 96.6,122.6, 123.2, 125.6, 126.7, 132.4, 134.5, 155.0, 166.7, 198.1
Elemental analysis: Calculated for C15H14N2O2: C, 70.85 %; H, 5.55 %; N, 11.02 %; Found: C, 70.67 %; H, 5.46 %; N, 11.13 %;
(4Z)-1,3-Dipropargyl)-4-(2-oxopropylidene)-1,3,4,5-tetrahydro-2H-1,5-benzodiazepin-2-one, 2b
This compound was obtained in 25% yield;
Melting Point: 173-176 °C
1H-NMR (250 MHz, CDCl3): δ= 1.9(t, 1H, 4J=2.6 Hz, 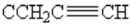 ), 2.1 (s,3H,CH3), 2.3 (t,1H, 4J=2.4 Hz,
), 2.1 (s,3H,CH3), 2.3 (t,1H, 4J=2.4 Hz, 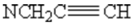 ), 3.3 (t, 1H, 4J=7 Hz,
), 3.3 (t, 1H, 4J=7 Hz, 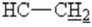 ), 4.2(HAHB, 2J =17.4 Hz,
), 4.2(HAHB, 2J =17.4 Hz, 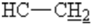 ),4.7 (d,2H,2J=2.5 Hz,
),4.7 (d,2H,2J=2.5 Hz, 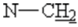 ),5.21(s,1H,
),5.21(s,1H, 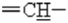 ),7.1-7.3(m,4H, HArom), 12.6(s,1H,NH).
),7.1-7.3(m,4H, HArom), 12.6(s,1H,NH).
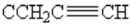 ), 2.1 (s,3H,CH3), 2.3 (t,1H, 4J=2.4 Hz,
), 2.1 (s,3H,CH3), 2.3 (t,1H, 4J=2.4 Hz, 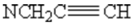 ), 3.3 (t, 1H, 4J=7 Hz,
), 3.3 (t, 1H, 4J=7 Hz, 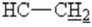 ), 4.2(HAHB, 2J =17.4 Hz,
), 4.2(HAHB, 2J =17.4 Hz, 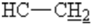 ),4.7 (d,2H,2J=2.5 Hz,
),4.7 (d,2H,2J=2.5 Hz, 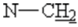 ),5.21(s,1H,
),5.21(s,1H, 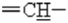 ),7.1-7.3(m,4H, HArom), 12.6(s,1H,NH).
),7.1-7.3(m,4H, HArom), 12.6(s,1H,NH).13C-NMR (62.9 MHz, CDCl3): δ= 16.0, 29.9, 38.6, 44.7, 93.5 122.7, 123.3, 125.8, 125.8, 127.0,131.9 134.2, 156.2, 166.9, 198.1
Elemental analysis: Calculated for C18H16N2O2: C, 73.95 %; H, 5.52 %; N, 9.58 %; Found: C, 73.82 %; H, 5.58 %; N, 9.66 %;
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6References
- El Abbassi, M.; Essassi, E.M.; Fifani, J. Tetrahedron Lett. 1987, 28, 1389.
- Djerrari, B.; El Abbassi, M.; Essassi, E.M.; Fifani, J. Tetrahedron Lett. 1989, 30, 7069.
© 2006 MDPI. All rights reserved.