Abstract
In this paper we propose the synthesis of 1-(2,4-dimethoxyphenyl)-4-(4-nitrophenyl)-3-phenoxyazetidin-2-one as a new monocyclic β-lactam. Its structure has been confirmed by IR, 1H-NMR, 13C-NMR and Mass spectroscopic data. In addition to its synthesis we present AM1 calculations to characterize the physical properties of the molecule.
Introduction:
It is generally appreciated that β-lactam based antibiotics have a limited future given increased resistance demonstrated by many strains of bacteria [1]. There is an urgent need for the development of new classes of antibiotic and many laboratories have made significant progress in this area, especially with the introduction of peptide-based agents [2]. The enchanted β-lactams has had a variety of medicinal applications. In addition to the well-known penicillins and cephalosporins antibiotics, a number of other biologically relevant enzymes have been targeted by β-lactams [3]. The need for potent and effective β-lactam antibiotics as well as more effective β-lactamase inhibitors has motivated chemists to design new β-lactams [4]. These compounds have served as starting materials in the preparation of various heterocyclic compounds of biological significance [5]. Substituted hydroxy β-lactams have been the starting materials in the semi-synthesis of paclitaxel (Taxol) and docetaxel (Taxotere) [6]. The medicinal use of some β-lactams as therapeutic agents for lowering plasma cholesterol levels has been documented [7]. Studies of human leukocyte elastase inhibitory mechanisms and the biological activity of this class of compounds have also been published [8]. There have been a few other remarkable developments, such as catalytic asymmetric [9] and polymer-supported [10] synthesis of β-lactams. In the mid 1970s, a new class of β-lactam antibiotics characterized by a single monocyclic structure, called monocyclic β-lactams [11].6 These are molecules which do not contain a ring fused the β-lactam ring [12].7 Some monocyclic β-lactams containing the phthalimido group have shown good antibacterial activity [13].8 As a result of this general trend of β-lactam use, the searches for clinically useful β-lactams that are antibacterial and have other medically important properties will continue [14,15,16].
Results and Discussion:
Treatment of 4-nitrobenzaldehyde 1 with 2,4-dimethoxyanillin 2 in ethanol under reflux condition afforded the desired Schiff base 3 as orange crystals in 85% yield. The [2+2] cycloaddition of this imine with the ketene (Staudinger reaction) generated in situ from phenoxyacetyl chloride in the presence of triethylamine in dry dichloromethane afforded the 1-(2,4-dimethoxyphenyl)-4-(4-nitrophenyl)-3-phenoxyazetidin-2-one 5 as a new monocyclic β-lactam (Scheme 1). This [2+2] cycloaddition (Staudinger reaction) afforded the cis stereoisomer. The coupling constants for H3 and H4 were about 5 Hz which is consistent with this kind of geometry. The cis geometric isomer of the product was also confirmed by theoretical calculations. Then the optimized structure of the molecule was calculated.
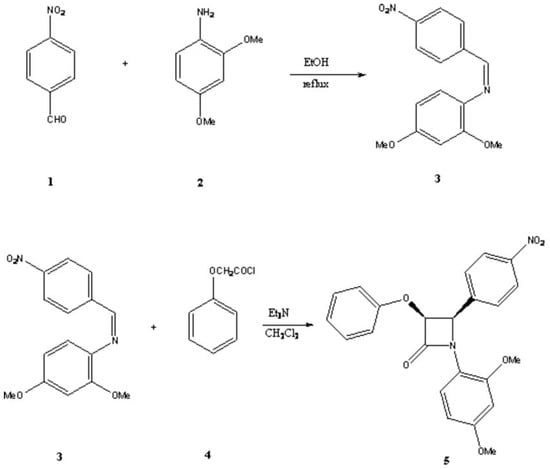
Scheme 1.
All calculations in this work where carried out with the AM1 level of theory using the GAUSSIAN 03 [17] suite of programs. More information about these methods is available elsewhere [18]. Figure 1 presents the optimized structure of the molecule with bond lengths and bond angles shown, also shown are the theoretical spectra, where frequencies are shown in cm−1, and IR intensities in KM/mol, and broadened theoretically via Doppler Broadening.
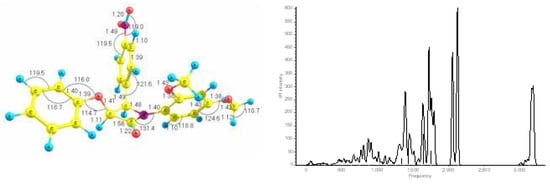
Figure 1.
AM1 optimized structure with theoretical spectrum, where bond lengths are in angstroms (Å), bond angles are in degrees (°), IR intensities in KM/mol, and frequencies in cm−1.
Table 1 shows the thermodynamic properties for the complex in figure 1 where T (temperature in K), S (entropy in J mol−1 K−1), Cp (heat capacity at constant pressure in kJ mol−1 K−1), and ΔH=H° - H°298.15 (enthalpy content, in kJ mol−1), T1=100 K, T2=298.15 K, and T3=1000 K calculated AM1 frequencies. The fits were performed according to the equations implemented by the National Institute of Standards and Technology (NIST) [19]. These equations have been very good at predicting physical properties of various molecules, as we have tested in the past [20,21,22,23].

Table 1.
Thermodynamic Parameters where T (temperature in K), S (entropy in J mol−1 K−1), Cp (heat capacity at constant pressure in kJ mol−1 K−1), and ΔH=H° - H°298.15 (enthalpy content, in kJ mol−1), T1=100 K, T2=298.15 K, and T3=1000 K calculated AM1 frequencies.
Experimental
General
All needed chemicals were purchased from Merck, Fluka chemical companies. Dichloromethane and triethylamine were dried by distillation over CaH2 and then stored over molecular sieve 4Å. IR spectra were run on a Shimadzu FT-IR 8300 spectrophotometer. 1H-NMR and 13C-NMR spectra were recorded in DMSO-d6 and CDCl3 using a Bruker Avance DPX instrument (1H-NMR 250 MHz, 13C-NMR 62.9 MHz). Chemical shifts were reported in ppm (δ) downfield from TMS. The mass spectra were recorded on a Shimadzu GC-MS QP 1000 EX instrument. Melting points were determined in open capillaries with Buchi 510 melting point apparatus and are not corrected. Thin-layer chromatography was carried out on silica gel 254 analytical sheets obtained from Fluka. Column chromatography was carried out on silica gel 60 Merck (230-270 mesh).
Synthesis of (2,4-Dimethoxybenzylidene)-(4-nitroyphenyl) amine (3).
A mixture of 2,4-dimethoxy aniline (1.68g, 10mmol) and p-nitrobenzaldehyde (1.51g, 10mmol) was refluxed in ethanol for 5 hours. Then the solvent was evaporated under reduced pressure. The crude product was recrystalized from cold methanol to give pure Schiff base 3 as orange crystals. (2.57g, 90%).
IR (KBr) (cm−1): 1612 (C=N).
1H-NMR (250 MHz, CDCl3): δ= 3.83 (OMe, s, 3H), 3.89 (OMe, s, 3H), 6.33-7.72 (aromatic protons, m, 3H), 8.26(ArH, d, 4H), 8.99 (HCN, s, 1H).
13C-NMR (62.9 MHz, CDCl3): δ= 55.55 (OCH3), 55.93 (OCH3), 99.54-142.31(Aromatic carbons), 155.85(PhCNO2),160.00(CN).
MS (m/z):286 (M+, 100%), 287 (M+1, 23.6%), 271 (43.5%), 225 (59.6%), 109 (49.3%).
1-(2,4-Dimethoxyphenyl)-4-(4-nitrophenyl)-3-phenoxy-2-azetidinone (5).
A solution of phenoxyacetyl chloride 4 (1.30 mmol) in dry CH2Cl2 (10 mL) was slowly added to a solution of (2,4-dimethoxybenzylidene)-(4-nitrophenyl) amine 3 (1.00 mmol) and freshly distilled triethylamine (3.00 mmol) in CH2Cl2 (15 mL) at –10 °C. The reaction mixture was then allowed to warm to room temperature and stirred overnight. Then it was washed with saturated sodium bicarbonate solution (20 mL), brine (20 mL), dried (Na2SO4) and the solvent was evaporated to give the crude product which was then purified by column chromatography over silica gel using 50:1 (n-Hexane/EtOAc).
Melting Point: 146-148 °C.
IR (KBr) (cm−1): 1758 cm –1(CO β-lactam).
1H-NMR (250 MHz, CDCl3): δ= 3.83, 3.89 (2OCH3, 2s, 6H), 4.61(H-4, d, 1H, J=5 Hz), 4.80(H-3, d, 1H, J=5.1Hz), 6.69-7.72 (ArH, m, 8H), 8.63(ArH, 2d, 4H).
13C-NMR (62.9 MHz, CDCl3): δ= 65.54, 65.60 (OCH3), 67.21 (C-4), 87.92 (C-3), 114.71-157.09 (aromatic carbons), 162.91(CO, β-lactam).
MS (m ⁄z):420 (M+), 421 (M+1), 286, 241, 179, 134.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6Acknowledgment
AAJ and SR thank the Shiraz University Research Council for financial support (Grant No.84-GR-SC-23). AFJ would like to thank the University of Arizona supercomputer center for over 200 hours of computer time for these calculations.
References
- Henry, C. M. Chem. Eng. News 2000(March), 41.
- Williams, D. H.; Bardsley, B. Angew. Chem. Int. Engl. 1999, 38, 1172.
- Banik, B. K.; Banik, I.; Becker, F. F. Bioorg. Med. Chem. 2005, 13, 3611–3622.b)β-Lactams: Synthesis, Stereochemistry, Synthons and Biological Evaluation; Current Medicinal Chemistry; Banik, B. K. (Ed.) Bentham Science, 2004; Vol. 11.
- Buynak, J. Curr. Med. Chem. 2004, 11, 1951–1964.
- Manhas, M. S.; Banik, B. K.; Mathur, A.; Vincent, J. E.; Bose, A. K. Tetrahedron 2000, 56, 5587–5601.
- Suffness, M. Taxol Science and Applications; CRC: Boca Raton, FL, 1995. [Google Scholar]
- Clader, J. W. J. Med. Chem. 2004, 47, 1–9. [CrossRef] [PubMed]
- Finke, P. E.; Shah, S. K.; Fletcher, D. S.; Ashe, B. M.; Brause, K. A.; Chandler, G. O.; Dellea, P. S.; Hand, K. M.; Maycock, A. L.; Osinga, D. G.; Underwood, D. J.; Weston, H.; Davies, P.; Doherty, J. B. J. Med. Chem. 1995, 38, 2449–2462. [CrossRef]
- France, S.; Weatherwax, A.; Taggi, A. E.; Lectka, T. Acc. Chem. Res. 2004, 37, 592–600. [CrossRef] [PubMed]
- Schunk, S.; Enders, D. J. Org. Chem. 2002, 67, 8034–8042. [CrossRef] [PubMed]
- Durcheimer, W.; Blumbach, J.; Lattrell, R.; Scheunamann, K. H. Angew. Chem. Int. Ed. Engl. 1985, 24, 25.
- Rode, J. E.; Dobrowolski, J. C. J. Molecular Struc. 2003, 651, 705. [CrossRef]
- Jarrahpour, A. A.; Shekarriz, M.; Taslimi, A. Molecules 2004, 9, 29–38.Jarrahpour, A. A.; Shekarriz, M.; Taslimi, A. Molecules 2004, 9, 939–948.Jarrahpour, A. A.; Zarei, M. Molecules 2006, 11, 49–58.
- Sandanayaka, V.P.; Prashad, A. S.; Yang, Y.; Williamson, T.; Lin, Y. I.; Mansour, T. S. J. Med. Chem. 2003, 46, 2569–2571.
- Buynak, J. D.; Rao, A. S.; Fod, G. P.; Carver, C.; Carver, C.; Adam, G.; Geng, B.; Bachmann, B.; Shobassy, S.; Lackey, S. J. Med. Chem. 1997, 40, 3423–3433. [CrossRef] [PubMed]
- Bonneau, P. R.; Hasani, F.; Plouffe, C.; Malenfant, E.; Laplante, S. R.; Guse, I.; Ogilvie, W. W.; Plante, R.; Davidson, W. C.; Hopkins, J. L.; Morelock, M. M.; Cordingley, M. G.; Deziel, R. J. Am. Chem. Soc. 1999, 121, 2965–2973. [CrossRef]
- Foresman, J. B.; Frisch, Æ. Exploring Chemistry with Electronic Structure Methods, 2nd edition; Gaussian, INC: Pittsburgh, PA, 1996. [Google Scholar]
- GAUSSIAN 03, Revision A.1, M. J. Frisch, et. Al., Gaussian, Inc.: Pittsburgh PA, 2003.
- Linstrom, P.J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook; NIST Standard Reference Database Number 69, July 2001, National Institute of Standards and Technology, Gaithersburg MD, 20899; ( http://webbook.nist.gov).
- Jalbout, A.F.; Solimannejad, M.; Labonowski, J.K. Chem. Phys. Letts. 2003, 379, 503.
- Jalbout, A.F.; Jiang, Z.-Y.; Quasri, A.; Jeghnou, H.; Rhandour, A.; Dhamelincourt, M.C.; Dhamelincourt, P.; Mazzah, A. Vib. Spect. 2003, 33, 21.
- Jalbout, A.F.; Nazara, F.; Turker, L. J. Mol. Struct. (THEOCHEM). 2004, 627, 1, (Invited Review).
- Jalbout, A.F.; Adamowicz, L.; Solimmanejad, M. Chem. Phys. Letts. 2006, in press.
© 2006 MDPI. All rights reserved.