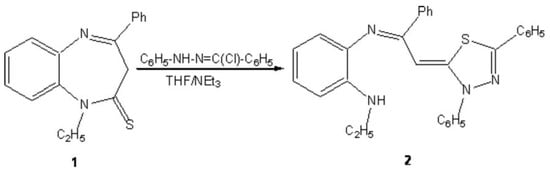
4-phenyl-1,5-benzodiazepine-2-thione are used as starting materials in the synthesis of several heterocyclic compounds for potential biological actvities.1-3 The synthesis of a new 1,3,4-thiadiazole derivative is reported.
To a solution of 0.01mol of 1-ethyl-4-phenyl-1,5-benzodiazepine-2-thione 1 [1] in 60 ml of tetrahydrofuran, 0,02 mol of hydrazonoyl chloride and 0.04 mol of triethylamine were added. Then, the mixture was refluxed for 12 hours. After cooling, salts are removed by filtration and solvent was evaporated under reduced pressure. The residue isolated was recrystallized from ethanol. The 1,3,4-thiadiazole derivative 2 was obtained in 60 % yield.
Melting point: 165 °C
1H-NMR (CDCl3, 250 MHz ): δ= 1,34 (t, J = 7,12 Hz, 3H), 3,36 (q, J = 7,12 Hz, 2H), 7,24 (s, 1H), 6,05-7,92 (m, 19H).
13C-NMR (CDCl3, 67.5 MHz ): δ= 15,24 (CH3), 38,94 (CH2), 90,19 (CH=), 110,00-130,26 (CHar), 130,53-142,35 (Car), 151,53 (C=N), 162,98 (C=N).
MS (EI): 474 [M]+
Elemental analysis: Calculated for C30H26N4S : C, 75.92%; H, 5.52%; N, 11.80%. Found: C, 75.97%; H, 5.46%; N, 11.84%;
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References:
- Essassi, E. M.; Salem, M. Bull. Soc. Chim. Belg. 1988, 97, 387.
- Essassi, E. M. Bull. Soc. Chim. Belg. 1994, 103, 679.
- Ghomsi, J.N.T; Ahabchane, N.H.; Essassi, E.M. Phosphorus Sulfur and Silicon 2004, 179, 353.
© 2006 MDPI. All rights reserved.