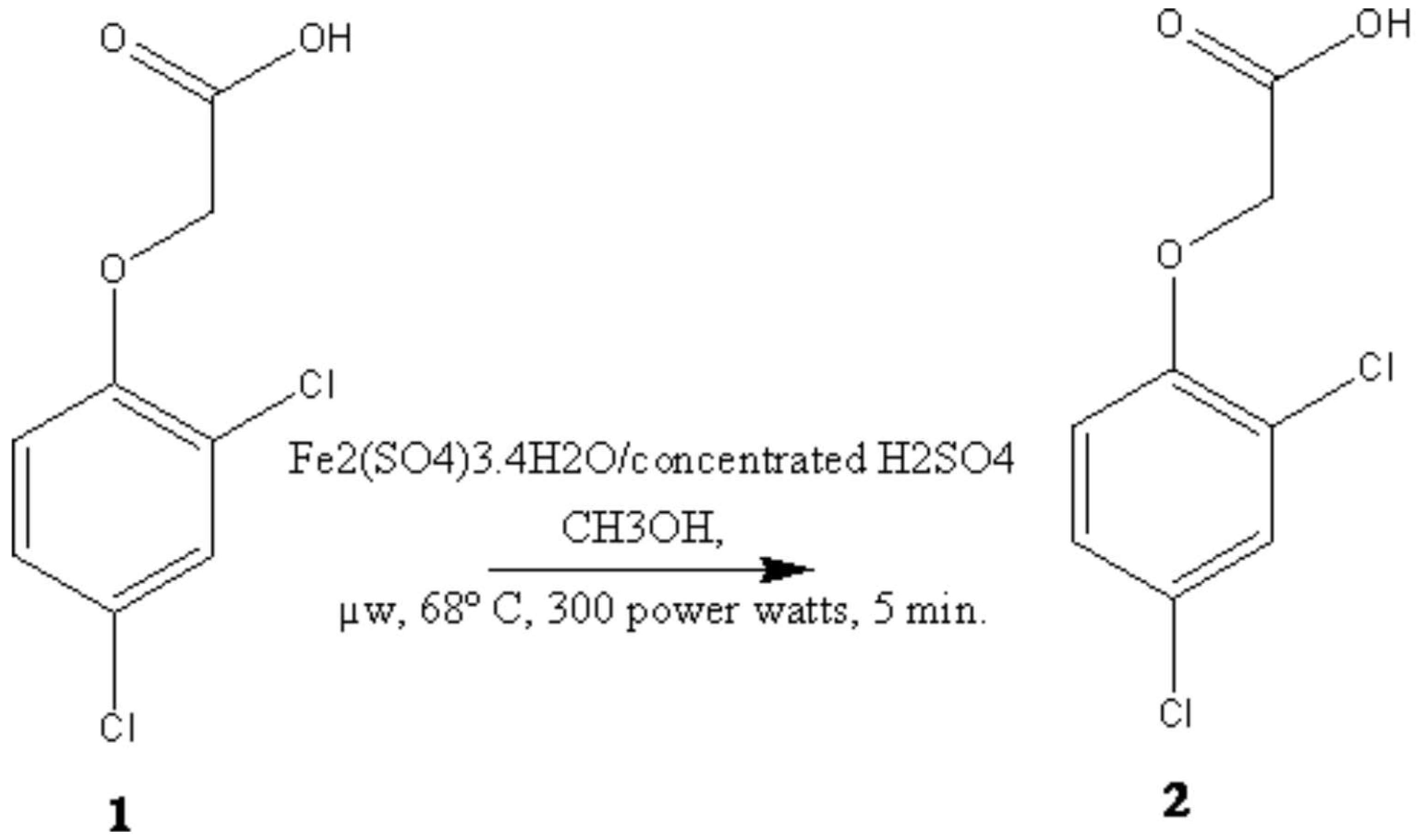
2, 4-D 1 (0.221 g, 1 m mole), Fe2(SO4)3.4H2O (0.423 g, 1 m mole) and conc. H2SO4 (0.098 mL) in absolute methanol (20 mL) was taken in RBF placed in a microwave oven and irradiated (300w, 67-68oC) for 5 min []. Upon completion of reaction (monitored by TLC), using petroleumether-ethylacetate (8:2) as the eluent solvent system. The reaction mixtures was allowed to attain room temperature, after the completion of reaction the solvent was removed by vacuum distillation and treated with cold water. The liquid product separated washed with water to furnish compound 2, yield 90%.
Melting point: 134-136 oC
IR (KBr) (cm-1): 1722 (>C=O of ester), 1225, 1044 (C-O-C), 3023 (C-H, aromatic ring), 1510 (C=C, aromatic ring), 825 (Ar-Cl).
1H-NMR (CDCl3-DMSO-d6) (400 MHz): δ= 6.70-7.90 (3H, m, Ar-H), 4.0 (3H, s, -COOCH3), 4.46 (2H, s, -CH2).
13C-NMR (CDCl3-DMSO-d6) (62.90 MHz): δ= 115.29-134.1 (aromatic carbons), 170 (>C=O of ester), 20 (-COOCH3), 35 (-CH2).
MS (m/z): 235 (M+) (C9H8O3Cl2+), 204 (C8H5O2Cl2+), 176 (C7H5OCl2+), 59 (C2H3O2+), 31 (CH3O+).
Elemental Analysis: Calculated for C9H8O3Cl2: C 45.95, H 3.40; found: C 45.98, H 3.43.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
The authors thank the Veer Narmad South Gujarat University, Surat for providing research facilities and one of the author (Krunal Desai) is thankful to the Gujarat Council On Science & Technology, Gandhinagar for financial support (Grant No. GUJCOST/200389/MRP/2003-04/10689). Authors are also thankful to CDRI, Lucknow for providing spectral and analytical data of the compounds. Authors are also thankful to Board of Radiation and Isotope Technology (BARC), Navi Mumbai.
References
- Desai, K. G.; Desai, K. R. Indian J. Chem. (Sec-B). 2005. (In Press)
- Sample Availability: Available from MDPI.
© 2006 MDPI. All rights reserved.