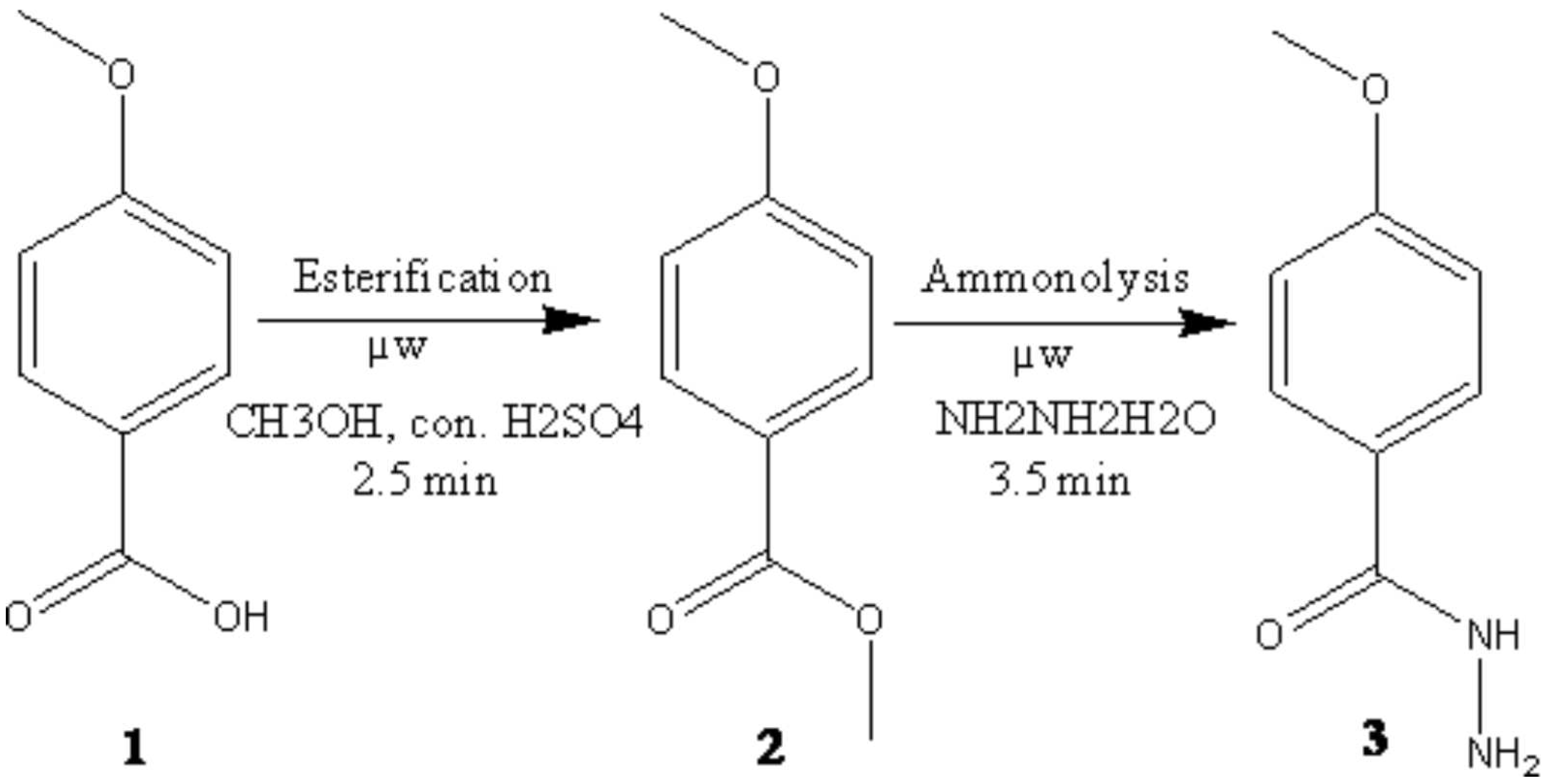
p-methoxy benzoicacid 1 (1.67 g, 0.01 mole) and catalytic amount of conc. H2SO4 (2-3 drops) in absolute methanol (30 mL) was taken in RBF placed in a microwave oven and irradiated (400w, 61-62oC) for 2.5 min [1]. Upon completion of reaction (monitored by TLC), using petroleumether-ethylacetate (8:2) as the eluent solvent system. The reaction mixtures was allowed to attain room temperature and treated with cold water. The solid separated was filtered, washed with water and recrystallised from ethanol to furnish compound 2, yield 90%.
Methyl ester of p-methoxy benzoicacid 2 (1.66 g, 0.01 mole) and hydrazine hydrate (0.9 mL, 0.01 mole) in ethanol (20 mL) was taken in RBF placed in a microwave oven and irradiated (450w, 76-78oC) for 3.5 min [1]. After completion of reaction (monitored by TLC), using petroleumether-ethylacetate (8:2) as the eluent solvent system. The mixture was cooled and the resulting solid was filtered, dried and recrystallized from ethanol to get compound 3, yield 83%.
Melting point: 88 oC 2 and 136 oC 3.
IR (KBr) (cm-1): 2 1722 (>C=O of ester), 1225, 1044 (C-O-C), 3023 (C-H, aromatic ring), 1510 (C=C, aromatic ring), 2825 (-OCH3). 3 1661 (>C=O of amide), 3355, 3382 (-NHNH2), 2837 (-OCH3), 3028, 1522 (C-H, C=C, aromatic ring).
1H-NMR (CDCl3-DMSO-d6) (400 MHz) δ (ppm): 2 6.70-7.90 (4H, m, Ar-H), 4.0 (3H, s, -COOCH3), 3.87 (3H, s, -OCH3). 3 6.85-7.96 (4H, m, Ar-H), 4.41 (2H, s, -NH2), 7.85 (1H, s, -CONH-), 3.89 (3H, s, -OCH3).
13C-NMR (CDCl3-DMSO-d6) (62.90 MHz) δ (ppm): 2 56.60 (-OCH3), 115.29-134.1 (aromatic carbons), 170 (>C=O of ester), 20 (-COOCH3). 3 57.68 (-OCH3), 117.31-130.0 (aromatic carbons), 162 (>C=O of amide).
MS (m/z): 2 166 (M+) (C9H10O3+), 135 (C8H7O2+), 107 (C7H7O+), 59 (C2H3O2+), 31 (CH3O+). 3 166 (M+) (C8H10O2N2+), 135 (C8H7O2+), 107 (C7H7O+), 31 (N2H3+), 16 (NH2+).
Elemental Analysis: Calculated for 2 C9H10O3: C 65.06, H 6.02. Found: C 65.10, H 6.00.
Elemental Analysis: Calculated for 3 C8H10N2O2: C 72.18, H 6.02, N 16.86. Found: C 72.20, H 6.04, N, 16.89.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
The authors thank the Veer Narmad South Gujarat University, Surat for providing research facilities and one of the author (Krunal Desai) is thankful to the Gujarat Council On Science & Technology, Gandhinagar for financial support (Grant No. GUJCOST/200389/MRP/2003-04/10689). Authors are also thankful to CDRI, Lucknow for providing spectral and analytical data of the compounds.
References
- Desai, K. G.; Desai, K. R. Indian J. Chem. (Sec-B). 2005. (In Press)
- Sample Availability: Available from MDPI.
© 2006 MDPI. All rights reserved.