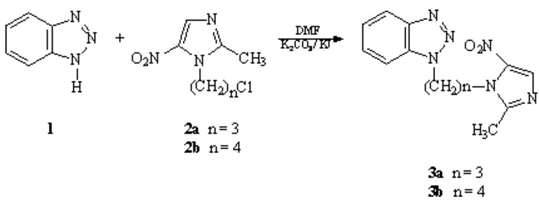
The title compounds were obtained in reaction 1,2,3-benzotriazole (1) with 1-(3-chloropropyl)- (2a) and 1-(4-bromobutyl)-2-methyl-5-nitro-1H-imidazole (2b) [1].
The derivatives of 5-nitroimidazole show various interesting biological properties. They have good chemotherapeutic activity [2], are potent anti-bacterial agents [2] and are very effective against various infections especially antiprotozoic [3].
1-[3-(2-methyl-5-nitro-1H-imidazol-1-yl)propyl]- 1H-1,2,3-benzotriazole (3a)
A mixture of 1,2,3-benzotriazole (1) (commercial product) (0.76 g, 6.38 mmol), 1-(3-chloro- propyl)-2-methyl-5-nitro-1H-imidazole (1.58 g, 7.76 mmol) (2a) [1] and powdered anhydrous K2CO3 (2 g) and a catalytic amount of KI in dry DMF (25 mL) was stirred at room temperature for 24 h. The resulting solution was poured into water (100 mL) and precipitated solid was filtered off and washed with water. The crude product was purified by recrystallization from isopropyl alcohol giving 1-[3-(2-methyl-5-nitro-1H-imidazol-1-yl) propyl]-1H-1,2,3-benzotriazole (3a) as colorless needles (1.45 g, 79.5%).
Melting point: 167-169°C
1H-NMR (CDCl3, 80 MHz ): δ= 8.10 (dd, 1H, aromatic H, J = 8.96 Hz , J = 1.21 Hz), 7.80 (s, 1H, CH imid.), 7.32-7.57 (m, 3H, aromatic H), 4.70 (t, 2H, CH2 CH2-N-benzotriazole, J = 6.40 Hz), 4.04 (t, 2H, CH2 CH2-N-imid., J = 7.12 Hz), 2.66-2.35 (m, 2H, CH2 CH2 CH2 ), 2.34 (s, 3H, CH3),
MS, (70eV) m/z (%): 286 (7.0), 269 (5.0), 171 (100).
IR (KBr, cm-1): 1537 and 1336 (NO2), 1495 (CH2), 1292 (C-N).
Elemental Analysis: Calculated for C13H14N6O2 (286.29): C 54.54, H 4.93, N 29.36; found C 54.23, H 4.87, N 29.02.
1-[3-(2-methyl-5-nitro-1H-imidazol-1-yl)butyl]-1H-1,2,3-benzotriazole (3b)
A mixture of 1,2,3-benzotriazole (1) (commercial product) (0.38 g, 3.19 mmol), 1-(3-bromo- butyl)-2-methyl-5-nitro-1H-imidazole (0.85 g, 3.43 mmol) (2b) [1] and powdered anhydrous K2CO3 (0.65 g) and a catalytic amount of KI in dry DMF (20 mL) was stirred at room temperature for 19 h. The resultating solution was poured into water (60 mL) and precipitated solid was filtered off, and washed with water. The crude product was purified by recrystallization from isopropyl alcohol giving 1-[3-(2-methyl-5-nitro-1H-imidazol-1-yl) butyl]-1H-1,2,3-benzotriazole (3b) as colourless needles (0.72 g, 75.2%).
Melting point: 168-170°C
1H NMR (CDCl3, 80 MHz ): δ= 7.93-7.34 (m, 4H, aromatic H), 7.66 (s, 1H, CH imid.), 4.81 (t, 2H, CH2 CH2-N-benzotriazole, J = 6.32 Hz), 3.91 (t, 2H, CH2 CH2-N-imid., J = 7.36 Hz ), 2.43-1.71 (m, 4H, CH2 CH2 CH2CH2 ), 2.37 (s, 3H, CH3).
IR (KBr, cm-1): 1539 and 1330 (NO2), 1496 (CH2) 1289 (C-N).
Elemental Analysis: Calculated C14H16N6O2 (300.32): C 55.99, H 5.37, N 27.98; found C 55.82, H 5.36, N 27.84.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6References
- Bourdais, J. Bull. Soc. Chim. 1968, 3246.
- Bhaumik, K.; Akamanchi, K.G. J. Heterocyclic Chem. 2004, 41, 51.
- Silvestri, R.; Artico, M.; Massa, S.; Marceddu, T.; de Montis, F.; La Colla, P. Bioorg. Med. Chem. 2000, 10, 253.
- Sample Availability: Available from MDPI.
© 2006 MDPI. All rights reserved.