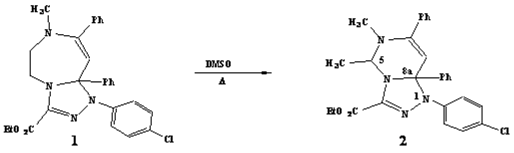
Compound 1 [1] (200 mg, 0.41 mmol) was refluxed in dimethylsulfoxide for 30 minutes. After cooling, the solvent was removed by evaporation under reduced pressure. The residue obtained was purified by chromatography on silica gel column (eluent: hexane/ethyl acetate 90/10), then the isolated product was recrystallized from ethanol to give 2 (130 mg, 65%).
Melting point: 130-132°C (Ethanol).
MS (m/z, %): 487 ([M+H]+, 100%).
1H-NMR (CDCl3, 250 MHz): δ= 1.11 (d, J = 6.90 Hz, 3H,CH3-C-5), 1.44 (t, J = 6.90 Hz, 3H, CH3-CH2-O), 2.58 (s, 3H, N-CH3), 4.41 (q, J = 6.90 Hz, 2H, CH3-CH2-O), 5.63 (s, 1H, H-C-8), 5.65 (q, J = 6.90 Hz, 1H, H-C-5), 6.80-7.70 (m, 14H, Ar-H).
13C-NMR and DEPT (CDCl3, 62.5 MHz): δ= 14.21, 19.07 (CH3-CH2-O, CH3-C-5,), 41.59 (N-CH3), 61.86 (CH3-CH2-O), 68.13 (C-5), 85.60 (C-8a), 101.33 (C-8).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References:
- Baouid, A.; Benharref, A.; Hasnaoui, A.; Lavergne, J.-P. Bull. Soc. Chim. Belg. 1994, 103(12), 743.
- Sample Availability: Available from MDPI.
© 2006 MDPI. All rights reserved.