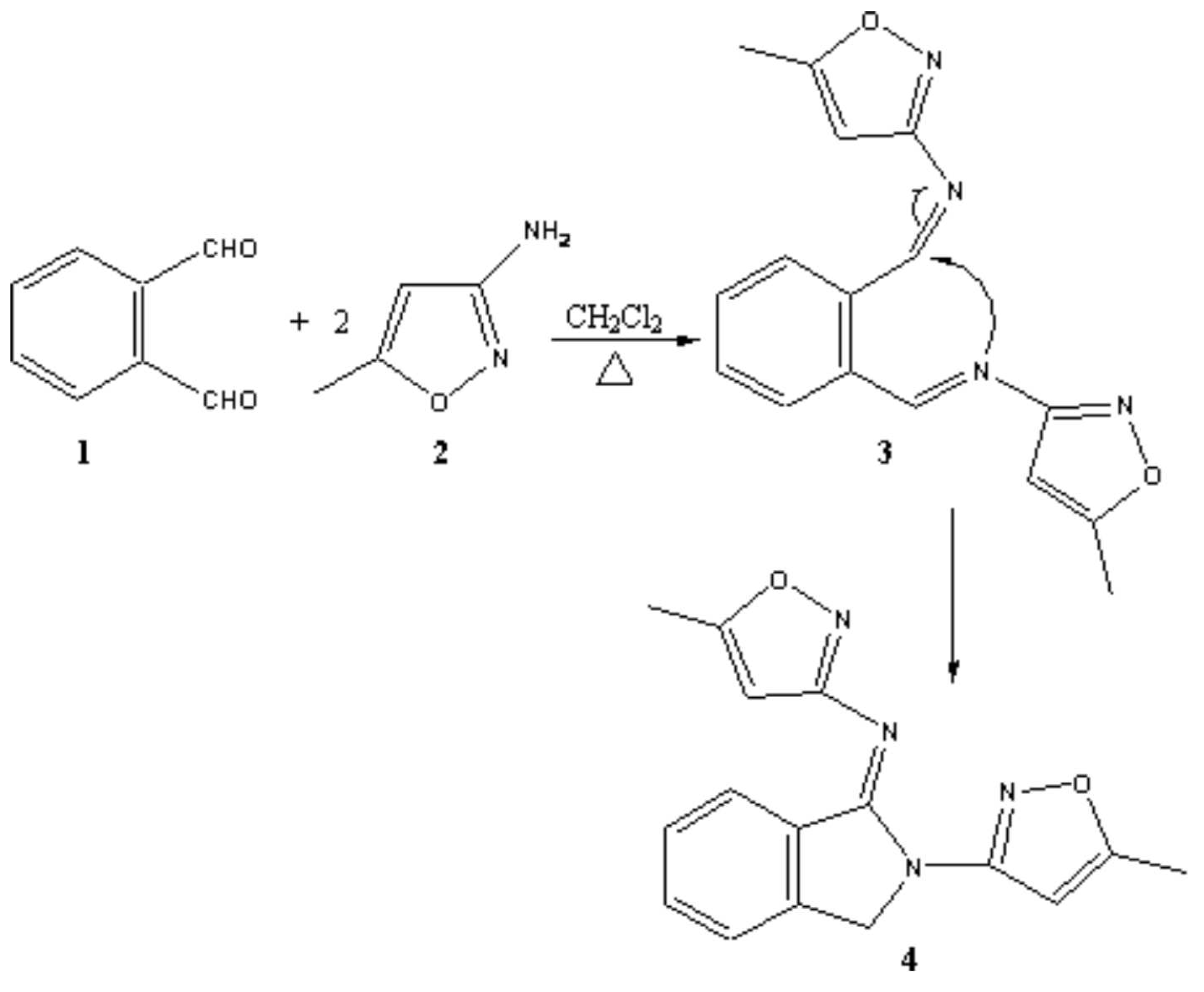
Synthesis of antifungal isoindole (5-methyl-isoxazole-3-yl)-[2-(5-methyl-isoxazol-3-yl)2,3-dihydro-isoindol-1-ylidene]amine
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References
- Takahashi, I.; Hatanaka, M. Heterocycles 1997, 45, 2475.
- Kundu, N. G.; Khan, M. W.; Mukhopadhyay, R. Indian Chem. Soc. 2001, 78, 671.
- Olmo, E.; Armas, M.; Ybarra, M.; López, J.; Oporto, P.; Giménez, A.; Deharo, E.; Feliciano, A. Bioorganic & Medicinal Chemistry Letters 2003, 13, 2769.
- Takahashi, I.; Miyamoto, R.; Nishiuchi, K.; Hatanaka, M.; Yamano, A.; Sakushima, A.; Sakushima, A.; Hosoi, S. Heterocycles 2004, 63, 1267.
- Cul, A.; Daïch, A.; Decroix, B.; Sanz, G.; Hijfte, L. Tetrahedron 2004, 60, 11029.
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.
Share and Cite
Al-Shihry, S.S. Synthesis of antifungal isoindole (5-methyl-isoxazole-3-yl)-[2-(5-methyl-isoxazol-3-yl)2,3-dihydro-isoindol-1-ylidene]amine. Molbank 2005, 2005, M447. https://doi.org/10.3390/M447
Al-Shihry SS. Synthesis of antifungal isoindole (5-methyl-isoxazole-3-yl)-[2-(5-methyl-isoxazol-3-yl)2,3-dihydro-isoindol-1-ylidene]amine. Molbank. 2005; 2005(6):M447. https://doi.org/10.3390/M447
Chicago/Turabian StyleAl-Shihry, Shar S. 2005. "Synthesis of antifungal isoindole (5-methyl-isoxazole-3-yl)-[2-(5-methyl-isoxazol-3-yl)2,3-dihydro-isoindol-1-ylidene]amine" Molbank 2005, no. 6: M447. https://doi.org/10.3390/M447
APA StyleAl-Shihry, S. S. (2005). Synthesis of antifungal isoindole (5-methyl-isoxazole-3-yl)-[2-(5-methyl-isoxazol-3-yl)2,3-dihydro-isoindol-1-ylidene]amine. Molbank, 2005(6), M447. https://doi.org/10.3390/M447




