In continuation of previous work on the polyfunctionally substituted 1H-pyrazolo[4,3-e][1,2,4]triazine [1,2] we have reported here preparation of 3-nitropyridin-2-yl hydrazone of 5-acetyl-3-phenyl-1,2,4-triazine 3 being valuable intermediate for the synthesis of nitro derivative of this ring system.
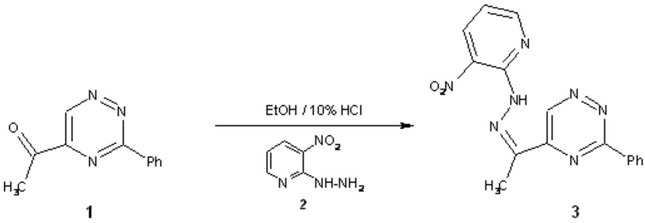
To a solution of 5-acetyl-3-phenyl-1,2,4-triazine 1(199 mg, 1mmol)[3] and 3-nitropyridin-2-ylhydrazine 2 (185 mg, 1.2 mmol) in ethanol (30 ml) 10% HCl (0.5 ml) was added. The resulting reaction mixture was heated at reflux for 5 min and then was stirred at room temperature for 30 min. After that time the solvent was concentrated in vacuo. The solid was collected by filtration, washed with water and recrystallized from DMSO/water (1:1) to give compound 3 in 82% yield.
Melting Point: 255ºC.
1H-NMR (200 MHz, d6-DMSO): δ= 2.53 (s, 3H); 7.21-7.27 (m, 1H); 7.62-7.67 (m, 3H); 8.44-8.54 (m, 3H); 8.65-8.68 (m, 1H); 9.56 (s, 1H); 11.36 (s, 1H).
IR (CHCl3 film, cm-1): 3316; 3069; 1598; 1530; 1500; 1366; 1264; 1199; 1053; 754; 697.
MS-EI (m/z, %): 335 (1) [M+]; 189 (30); 180 (9); 179 (100); 157 (6); 143 (8); 133 (5); 128 (7); 103 (7); 102 (4); 77 (5).
HR-MS: Calculated for C16H13N7O2: 335.11307. Found: 335.11339.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.