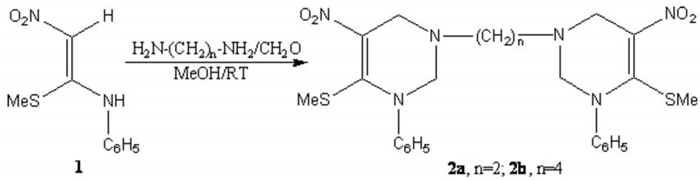
Bis-(1-phenyl-5-nitro-6-methylthio-1,2,3,4-tetrahydropyrimidinyl)ethane and Bis-(1-phenyl-5-nitro-6-methylthio-1,2,3,4-tetrahydropyrimidinyl)butane
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Urbanska, H.D.; Kartitzky, A.R.; Urbanski, T. Tetrahedron 1969, 25, 1617.
- Sone, M.; Tominaga, Y.; Matsuda, Y.; Kobayashi, G. Yakugaku Zasshi 1977, 97, 262, Chem. Abstr. 1977, 87, 53195 v.
- Vishwakarma, J.N.; Mofizuddin, M.; Ila, H.; Junjappa, H. J. Heterocyclic Chem. 1988, 25, 1387. [CrossRef]
- Karim, E.; Kishore, K.; Vishwakarma, J.N. J. Heterocyclic Chem. 2003, 40, 901–903. [CrossRef]
- Gompper, R.; Schaefer, H. Chem. Ber. 1967, 100, 591–604. [CrossRef]
© 2004 MDPI. All rights reserved
Share and Cite
Chanda, K.; Dutta, M.C.; Kishore, K.; Vishwakarma, J.N. Bis-(1-phenyl-5-nitro-6-methylthio-1,2,3,4-tetrahydropyrimidinyl)ethane and Bis-(1-phenyl-5-nitro-6-methylthio-1,2,3,4-tetrahydropyrimidinyl)butane. Molbank 2004, 2004, M367. https://doi.org/10.3390/M367
Chanda K, Dutta MC, Kishore K, Vishwakarma JN. Bis-(1-phenyl-5-nitro-6-methylthio-1,2,3,4-tetrahydropyrimidinyl)ethane and Bis-(1-phenyl-5-nitro-6-methylthio-1,2,3,4-tetrahydropyrimidinyl)butane. Molbank. 2004; 2004(1):M367. https://doi.org/10.3390/M367
Chicago/Turabian StyleChanda, Kaushik, Milan C. Dutta, Kaushal Kishore, and J. N. Vishwakarma. 2004. "Bis-(1-phenyl-5-nitro-6-methylthio-1,2,3,4-tetrahydropyrimidinyl)ethane and Bis-(1-phenyl-5-nitro-6-methylthio-1,2,3,4-tetrahydropyrimidinyl)butane" Molbank 2004, no. 1: M367. https://doi.org/10.3390/M367
APA StyleChanda, K., Dutta, M. C., Kishore, K., & Vishwakarma, J. N. (2004). Bis-(1-phenyl-5-nitro-6-methylthio-1,2,3,4-tetrahydropyrimidinyl)ethane and Bis-(1-phenyl-5-nitro-6-methylthio-1,2,3,4-tetrahydropyrimidinyl)butane. Molbank, 2004(1), M367. https://doi.org/10.3390/M367



