The importance of carbohydrates for asymmetric synthesis is well recognized [1,2,3,4].The reason that carbohydrates contain too many chiral centers and functional groups has limited their research about stereodifferentiating selection processes in asymmetric synthesis for a long time. During the past decades few carbohydrates have received increasing attention as stereodifferentiating auxiliaries in stereoselective syntheses [5]. Carbohydrates have been applied for stereo- and regioselective chemical reactions for last two decades. They could be used for biological recognition on membrane, and they play a prominent role in numerous biological processes such as tumor-cell growth [6], bacterial and viral infection or inhibition of glycosidases [2,7,8,9]. Glycosylamines are valuable intermediates in the preparation of nucleosides and drugs [10,11,12]. It has been proposed that when the Schiff base is derived from an optically active amine and an achiral aldehyde, the degree of distereoselectivity in the [2+2] cycloaddition varies [13,14]. The asymmetric Staudinger reaction utilizing 2,3,4,6-tetra-O-acetyl-β-D-galactopyranosylamine and 2,3,4,6-tetra-O-pivaloyl-β-D-galactopyranosylamine as the chiral auxiliary in the synthesis of 2-azetidinones has been reported by us and others [15,16,17,18]. We now report compound 6 as a new chiral auxiliary Schiff base for β-lactam syntheses.
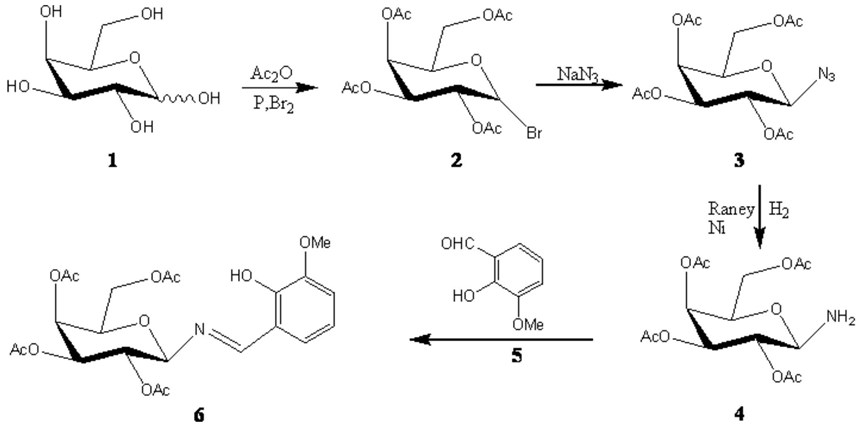
D-(+)-Glucose 1 was converted to 2, 3, 4, 6-tetra-O-acetyl-β-D-galactopyranosylbromide 2 by a reported method [18]. Treatment of 2 with NaN3 in 9:1 aceton-water afforded galactosylazide 3 in 95% yield. Reduction of 3 by hydrogenation in the presence of Raney Ni gave 2, 3, 4, 6-tetra-O-acetyl-β-D-galactopyranosylamine as a white solid which was recrystallized from ethanol. o-Vanillin 5 (0.87 g, 5.71 mmol) was added to a solution of 2,3,4,6-tetra-O-acetyl-β-D-galactosylamine 4 (2.00 g, 5.76 mmol) in ethanol (35 ml). The mixture was refluxed for 5 h. The resulting yellow solid N-(2-hydroxy-3-methoxybenzylidene) - 2,3,4,6-tetra-O-acetyl -β-D galactopyranosylamine 6 was collected in 90% yield by filtration.
= + 24.5 (c = 0.02, CHCl3)
Melting point: 180-182 °C.
IR (KBr, cm-1): 3357.8-3620.1 (OH); 1743.5 (C=O); 1633.6 (C=N) cm-1.
1H-NMR (250MHz, DMSO-d6): δ= 12.44 (OH, br, 1H); 8.53 (NCH, s, 1H); 7.30-6.82 (Ar-H, m, 4H); 3.84 (OCH3, s, 3H); 1.91-2.10 (4CH3CO, s, 12H),
13C-NMR (62.9 MHz, DMSO-d6): δ= 170.82-165.03 (C=O); 124.90-115.21 (Ar); 21.10-20.97(OCH3).
MS (m⁄z, %): 481 (2.00); 331 (2.10); 169 (21.60); 109 (18.10); 43 (100.00).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
The authors thank the Shiraz University Research Council for financial support (Grant No. 83-GR-SC-31 and 84-GR-SC-23).
References
- Kunz, H.; Pfrengle, W. J. Am. Chem. Soc. 1988, 110, 651. [CrossRef]
- Kunz, H.; Pfrengle, W. Tetrahedron 1988, 44, 5487. [CrossRef]
- Sharma, G V M; Reddy, G.; Radha Krishna, P. Tetrahedron Lett. 1999, 40, 1783.
- Totani, K.; Takao, K.; Tadano, K. Synlett 2004, 12, 2066.
- Kunz, H. Modern Amination Methods; Ricci, A., Ed.; WILEY-VCH: einheim, 2000; p. 103. [Google Scholar]
- Danishefsky, S. J.; Aِِِِِِِllen, J. R. Angew. Chem. Int. Ed. Engl. 2000, 39, 836.
- Pili, R.; Chang, J.; Patris, R.A.; Mueller, R. A.; Chrest, F. J.; Passaniti, A. Cancer Res. 1995, 55, 2920.
- Wonge, C. H.; Sears, P. Angew. Chem. Int. Ed. Engl. 1999, 38, 2300.
- Dotz, K.; Jakel, C.; Hasse, W. J. Organomet. Chem. 2001, 617-618, 119.
- Babiano, R.; Fuentes Mota, J. Carbohydr. Res. 1986, 154, 280.
- Cusack, N. J.; Hildick, B. J.; Robinson, D. H.; Rugg, P. W.; Shaw, G. J. Chem.Soc. 1973, 1720.
- Cusack, N. J.; Robinson, D. H.; Rugg, P. W.; Shaw, G.; Lofthouse, R. J. Chem. Soc. 1974, 73.
- Barton, D. H. R.; Getau-Olesker, A.; Anaya-Mateos, J.; Cleophax, J.; Gero, S. D.; Chiaroni, A.; Riche, C. J. Chem. Soc., Perkin Trans.1 1990, 3211.
- Jarrahpour, A. A.; Shekarriz, M.; Taslimi, A. Molecules 2004, 9, 939. [CrossRef] [PubMed]
- Jarrahpour, A. A.; Shekarriz, M.; Taslimi, A. Molecules 2004, 9, 29. [CrossRef] [PubMed]
- Krank, B.; Hebrault, D.; Schultz, M.; Kunz, H. Synlett 2003, 4, 671.
- Kunz, H.; Sager, W.; Schanzenbach, D.; Decker, M. liebigs Ann. Chem. 1991, 7, 649.
- Furniss, B. S.; Hannaford, A. J.; Smith, P. W. G.; Tartchel, A.R. Textbook of Practical Organic Chemistry (Vogel’s), 5th ed.; John Wiley & Sons, Inc.: New York, 1991; p. 648. [Google Scholar]
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.