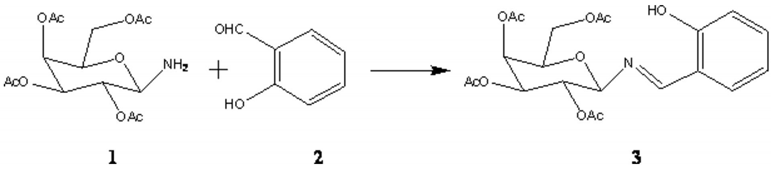
Synthesis of β-D galactopyranosyl amino-(N-salicylidene) - 2, 3, 4, 6-tetra-O-acetate as a new chiral Schiff base for asymmetric transformations
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References
- Kunz, H.; Pfrengle, W. Tetrahedron 1988, 44, 5487. [CrossRef]
- Sharon, N.; Lis, H. Chem. Eng. News 1981, 21, 21. [CrossRef]
- Kunz, H.; Pfrengle, W. Angew. Chem. Int. Ed. Engl. 1989, 28, 1067. [CrossRef]
- Kunz, H.; Pfrengle, W. J. Am. Chem. Soc. 1988, 110, 651. [CrossRef]
- Kunz, H.; Sager, W. Angew. Chem. Int. Ed. Engl. 1987, 26, 557. [CrossRef]
- Babiano, R.; Fuentes Mota, J. Carbohydr. Res. 1986, 154, 280.
- Cusack, N. J.; Hildick, B. J.; Robinson, D. H.; Rugg, P. W.; Shaw, G. J. Chem. Soc. 1973, 1720–1731.
- Cusack, N. J.; Robinson, D. H.; Rugg, P. W.; Shaw, G.; Lofthouse, R. J. Chem. Soc. 1974, 73–81.
- Kunz, H. Modern Amination Methods; Ricci, A., Ed.; WILEY-VCH: Weinheim, 2000; p. 103. [Google Scholar]
- Jarrahpour, A. A.; Shekarriz, M.; Taslimi, A. 123. Molecules 2004, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.
Share and Cite
Jarrahpour, A.A.; Alvand, P.; Arab, R.; Beheshti, A. Synthesis of β-D galactopyranosyl amino-(N-salicylidene) - 2, 3, 4, 6-tetra-O-acetate as a new chiral Schiff base for asymmetric transformations. Molbank 2005, 2005, M435. https://doi.org/10.3390/M435
Jarrahpour AA, Alvand P, Arab R, Beheshti A. Synthesis of β-D galactopyranosyl amino-(N-salicylidene) - 2, 3, 4, 6-tetra-O-acetate as a new chiral Schiff base for asymmetric transformations. Molbank. 2005; 2005(4):M435. https://doi.org/10.3390/M435
Chicago/Turabian StyleJarrahpour, A. A., P. Alvand, R. Arab, and A. Beheshti. 2005. "Synthesis of β-D galactopyranosyl amino-(N-salicylidene) - 2, 3, 4, 6-tetra-O-acetate as a new chiral Schiff base for asymmetric transformations" Molbank 2005, no. 4: M435. https://doi.org/10.3390/M435
APA StyleJarrahpour, A. A., Alvand, P., Arab, R., & Beheshti, A. (2005). Synthesis of β-D galactopyranosyl amino-(N-salicylidene) - 2, 3, 4, 6-tetra-O-acetate as a new chiral Schiff base for asymmetric transformations. Molbank, 2005(4), M435. https://doi.org/10.3390/M435



