O- and N-alkyl derivatives of pyrazolo[4,3-e][1,2,4]triazine, isolated from the cultural fluids of Pseudomonas fluorescens and Nostoc spongiaeforme exhibit antimicrobial and antitumor activity [1,2]. In this communication we report the synthesis of 5-methoxy-1,3-dimethyl-1H-pyrazolo[4,3-e][1,2,4]triazine (2) with the goal of performing structure-activity relationship studies.
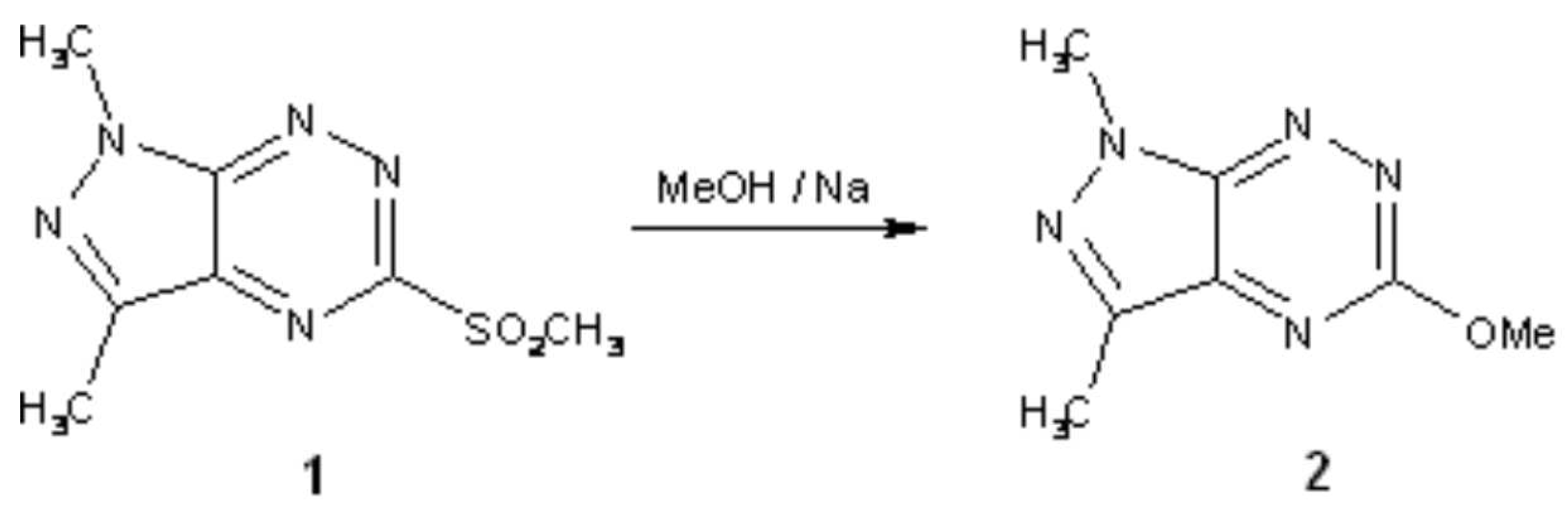
To a solution of the sulfone 1 (145 mg, 0.5 mmol) in absolute methanol (10 ml) metal sodium (35 mg, 1.5 mmol) was added. The reaction mixture was heated at reflux for 30 min. After usual work-up compound 2 was purified by column chromatography (Merck, silica gel 230-400 mesh) using CHCl3 as eluent. The 1,3-dimethyl-5-methoxy-1H-pyrazolo[4,3-e][1,2,4]triazine (2) was isolated in 96% yield.
Melting Point: 110°C.
1H-NMR (200 MHz, CDCl3): δ= 2.59 (s, 3H); 4.20 (s, 3H); 4.24 (s, 3H).
IR (KBr, cm-1): 2960; 1310; 1120; 1050.
MS- EI (m/z): 179[M+].
Elemental Analysis: Calculated for C7H9N5O: C, 46.93%; H, 5.02%; N, 39.10%. Found: C, 47.20%; H, 4.94%; N, 38.89%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Smirnov, V.V.; Kiprianowa, E.A.; Garagulya, A.D.; Esipov, S. E.; Dovjenko, S. A. FEMS Microbiology Lett. 1997, 153, 357.
- Hirata, K.; Nakagami, H.; Takashina, J.; Mahmud, T.; Kobayashi, M.; In, Y.; Ishida, T.; Miyamoto, K. Heterocycles 1996, 43, 1513.
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.
