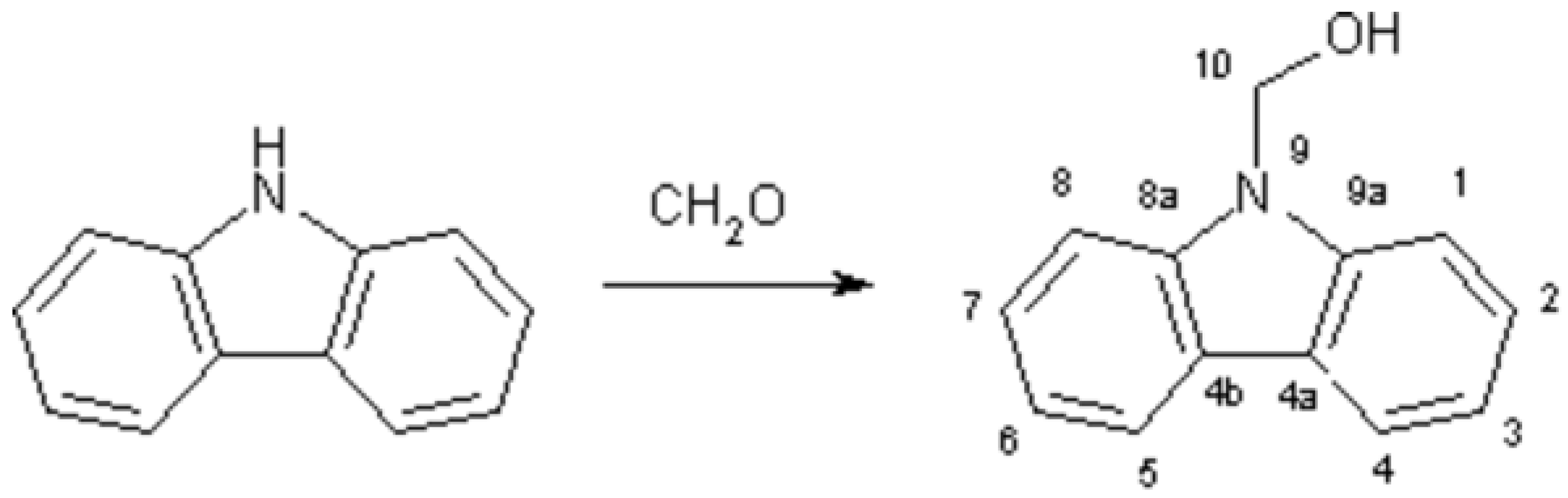
9H-Carbazole derivatives are known to have fluorescent properties and therefore they have exploitation in analytical chemistry, especially in bioanalytical chemistry. Carbazol-9-yl-methanol is a known agent in fluorescent analysis used with this aim.
Preparation of carbazol-9-yl-methanol is described only in patent literature [1] and in a journal difficult of access [2]. Title compound is stable only in alkaline alcoholic solutions and in acidic media turns to N,N´-biscarbazol-9-yl-methane [3].
To a mixture of carbazole (16,7 g, 0,1 mol) in ethanol (60 mL) is added potassium carbonate (10 g, 60 mmol) and the mixture is boiled under reflux for 5 min. with magnetic stirring. Then methanal (10 mL, 35 % aqueous solution, 0.1 mol) is added and heating is continued to dissolve all components. After next 10 min. the resulting reaction mixture is cooled, separated product is filtered off, dried and recrystallized from toluene. Yield 10.8 g ( 91.4 %).
M.p. 128-129 deg.C (toluene), 127-128 deg. C (EtOH) [1]; 128-129 deg. C (EtOH) [2].
TLC(CHCl3/EtOH=9:1): Rf 0.57.
1H NMR (DMSO-d6, 300 MHz): 1.91t (1H, OH), 5.77d (2H,CH2), 7.22t (1H,H-2), 7.45t (1H,H-3), 7.67d (1H,H-1,3J=8.4 Hz), 8.14d (1H,H-4,3J=7,8 Hz) ppm.
13C NMR (DMSO-d6, 75 MHz): 65.33 (CH2,1J=55.11 Hz), 109.93 (C-1,C-8,1J=163.17 Hz, 2J=9.06 Hz), 119.41 (C-3,C-6,1J=161.16 Hz, 2J=8.06 Hz), 120.24 (C-4,C-5,1J=160.15 Hz, 2J=8.06 Hz), 122.62 (C-4a, C-4b, 2J=5.8 Hz), 125.72 (C-2,C-7,1J=161.15 Hz, 2J=7.05 Hz), 139.78 (C-8a, C-9a, 2J=4.3 Hz) ppm.
Anal. calc. for C13H11NO (197.24): C 79.17, H 5.62, N 7.10; found: C 79.01, H 5.66, N 7.24 %.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements:
The authors would like to thank Slovak Grant Agency (financial support No. 1/9254/02 and 1/0058/03).
References and Notes
- Lange, M. Ger. 256757 (1912). Chem. Abstr. 1913, 7, 2454. [Google Scholar]
- Lopatinskii, V.P.; Sirotkina, E.E. Metody Poluch. Khim. Reakt. Prep. 1964, 88, Chem. Abstr. 1966, 64, 15823h.
- Traven, V.F.; Smrček, V.A.; Stepanov, B.I. Khim. Geterotsikl. Soedin. 1967, 3, 568.
© 2004 MDPI. All rights reserved.