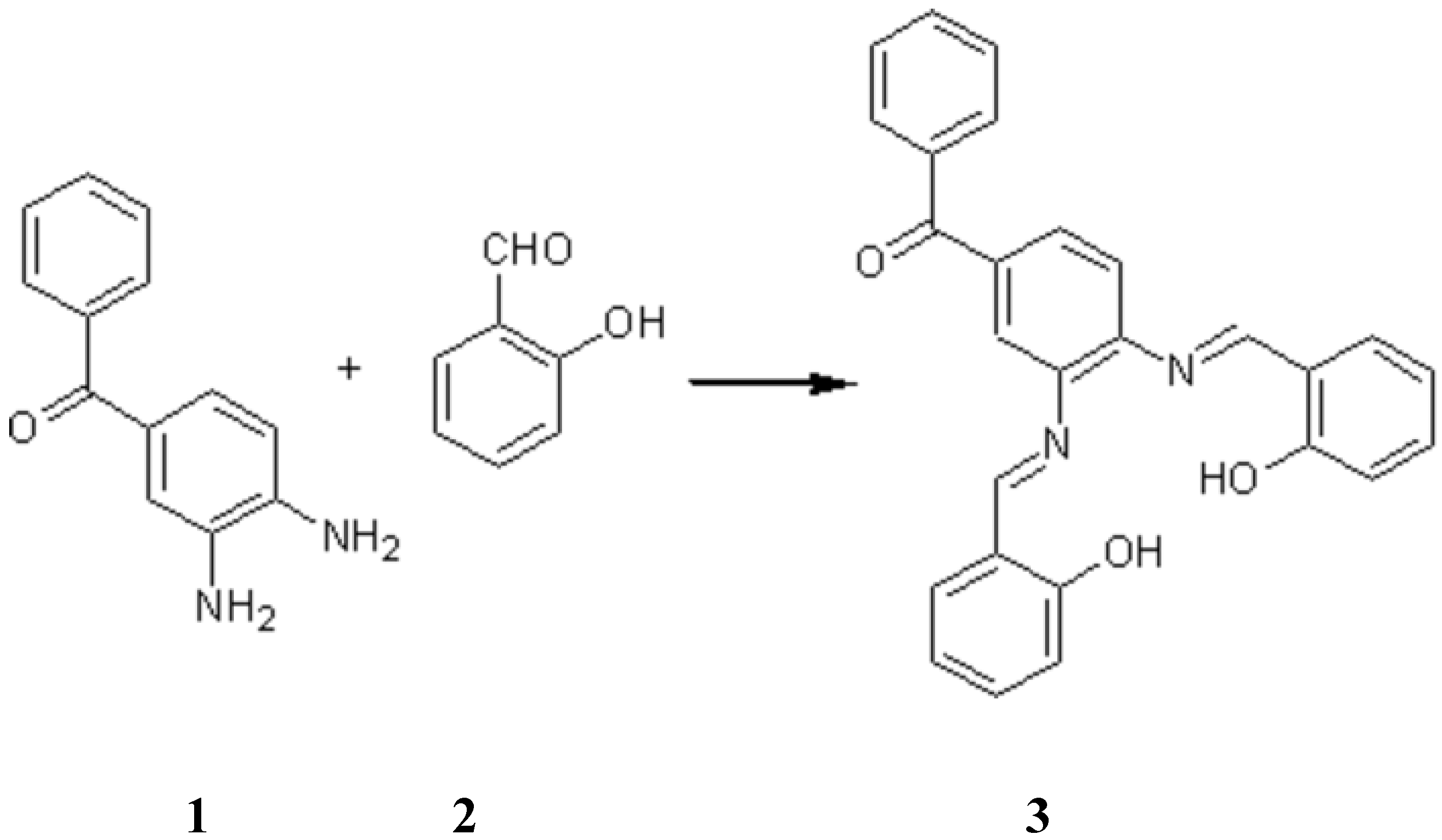
A mixture of (3,4-diaminophenyl) phenyl methanone 1 (2.12 g, 10.00 mmol), 2-hydroxybenzaldehyde 2 (2.44 g, 20.00 mmol) and sodium sulfate (5.00 g) in dry dichloromethane (50.00 ml) was stirred at room temperature for five hours. The suspension was filtered and washed with dichlormethane. The solvent was evaporated and compound 3 was formed as an orange solid which was recrystalized from ethanol (3.41 g, 81.30%).
m.p. 159-161oC.
IR (KBr) (cm-1): 1600 (C=N), 1650 (CO), 3100-3300 (OH)
1H-NMR (CDCl3) (250 MHz)δ(ppm): 6.95-7.76 (16H, m, 4Ph), 8.59 (2H, s, N=CH), 12.71 (2H, br, OH)
13C-NMR (CDCl3) (62.90 MHz)δ(ppm): 115.00-144.25 (aromatic carbons), 159.45 (C-OH), 163.05 (C=N), 193.00 (CO).
MS (m/z, %): 420 (M +, 8.40), 314 ( PhCOPh(N)(N=CHPhOH, 100.00), 237 (PhCOPh(N=CH)2, 70.00) , 209 (PhCOPh(N)2, 23.50 ), 182 (PhCOPh, 5.00), 105 (PhCO, 41.20), 77 (PhH)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
The authors thank the Shiraz University Research Council for financial support (Grant No. 81-SC-1540-C220).
- Sample availability: Available from MDPI
© 2004 MDPI. All rights reserved.