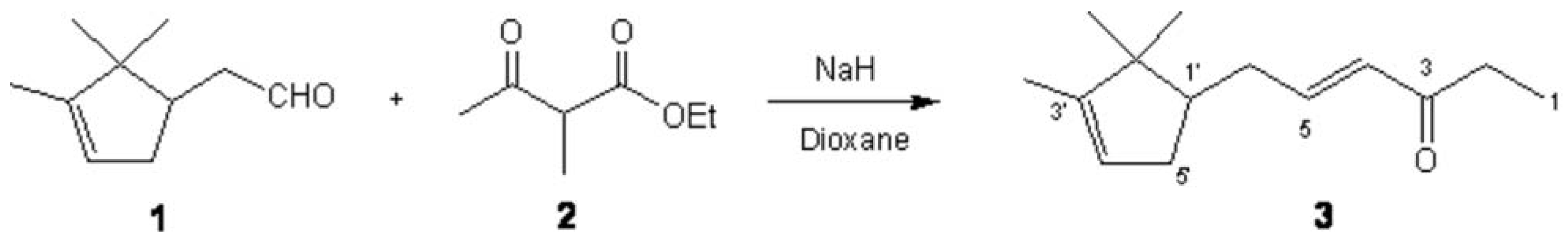
Ethyl 2-methylacetoacetate (2) (727 mg, 4.53 mmol) was added to a stirred solution of NaH (184 mg, 4.60 mmol) in dioxane (25 mL). Then campholenic aldehyde (1) (707 mg, 3.72 mmol) was added and the mixture refluxed for 15 h. Then 2N HCl (15 mL) was added and the mixture extracted with Et2O (3×15 mL). The combined organic layers were washed with 2N HCl (2×15 mL), saturated Na2CO3 (2×15 mL) and brine (3×15 mL). The organic phase was dried over anhydrous Na2SO4 and the solvent evaporated under reduced pressure to yield a residue (900 mg) which was purified by distillation under reduced pressure to give the title compound 3 (505 mg, 2.63 mmol, 58%).
IR (neat, ν, cm-1): 1700, 1676 (CO), 3036, 1631, 984 (C=C).
1H NMR (300 MHz, CDCl3, δ, ppm): 0.81 (3H, s, Me-2’), 1.00 (3H, s, Me’-2’), 1.10 (3H, t, J=7.4 Hz, H-1), 1.61 (3H, br s, Me-3’), 1.77–2.42 (5H, m, H-5’, H-1’, H-6), 2.57 (2H, q, J=7.4 Hz, H-2), 5.22 (1H, br s, H-4’), 6.14 (1H, dt, J=15.8 Hz, 1.4 Hz, H-4), 6.85 (1H, dt, J=15.8 Hz, 7.3 Hz, H-5).
13C NMR (75 MHz, CDCl3, δ, ppm): 8.13 (C-1), 33.46* (C-2), 201.04 (C-3), 130.50 (C-4), 146.89 (C-5), 33.14* (C-6), 49.27 (C-1’), 46.90 (C-2’), 148.30 (C-3’), 121.45 (C-4’), 35.42 (C-5’), 19.72 (Me-2’), 25.80 (Me’-2’), 12.54 (Me-3’).
*These signals may be interchanged.
MS (70 eV, m/z): 206 (M+, 3%), 191 (M+–Me, 2), 177 (M+–Et, 4), 173 (5), 163 (4), 149 (M+–COEt, 5), 145 (7), 136 (7), 121 (11), 108 (C8H12+, 53), 98 (56), 93 (55), 79 (33), 67 (29), 57 (C3H5O+, 100), 41 (49).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements:
We wish to thank the Ministerio de Ciencia y Tecnología for financial support (R+D Project PPQ2000-1665) and the Ministerio de Educación, Cultura y Deporte for a fellowship to J. M. Castro.
© 2004 MDPI. All rights reserved.
