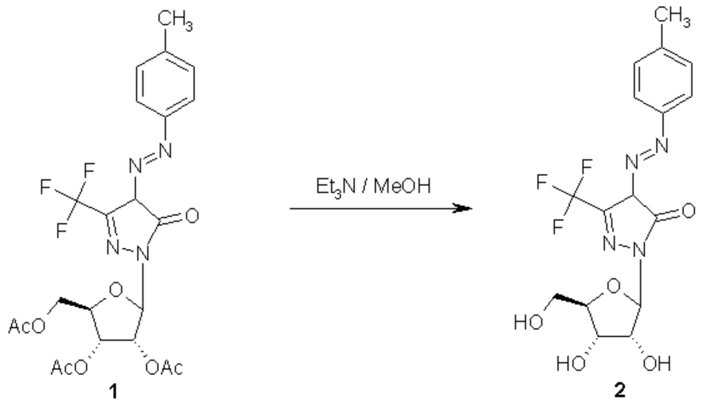
The desired compound 2 was obtained by complete deprotection of the acetylated nucleoside 1 [1] using triethylamine [2]. To a solution of 1 (0.8g, 1.5 mmol) in methanol (25 ml) was added triethylamine (2 ml). The mixture was stirred at room temperature and the reaction was followed by tlc. After complete deprotection (24 hours), the reaction mixture was evaporated and coevaporated with methanol (3 x 30 ml), then chromatographed over silica gel using CH2Cl2/MeOH (95:5 v/v) to give 0.55 g (90%) of 2 as yellow powder.
Rf: 0.30 (CH2Cl2/MeOH, 95/5 v/v).
UV (lmax , 95% ethanol): 384nm
IR (KBr, cm-1): 3414 (OH), 1662 (CO pyrazolone).
MS (m/z): 402.
1H-NMR (250 MHz, DMSO-d6): 2.38(s, 3H, CH3); 2.40(s, 1H, CH); 3.73(dd, 1H, H-5` J5`,4`=2.4 Hz) 3.91-3.96(dd, 1H, H-5`` J5``,4`=2.4 Hz); 4.23-4.24(m, 1H, H-4`); 4.53(t, 1H, H-3` J3`,2`=3.66 Hz); 4.75(t, 1H, H-2` J2`,3`=5.13 Hz); 5.94(d, 1H, H-1`, J1`,2`=4.92 Hz); 7.21-7.37(m, 4H, aromatic CH).
13C-NMR (75 MHz, DMSO-d6): 22.0(CH3); 48.9(CH); 63.08(C-5`), 71.71(C-3`); 73.90(C-2`); 85.63(C-4`); 88.08(C-1`); 116.9 (2 aromatic carbons), 121.0, 122.0 (2 aromatic carbons), 130.4, 144.0 (2 quaternary aromatic carbons); 137.7(q, CF3); 148.5(C=N); 173.5(CO).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Haikal, A.; Zohdi, H. F.; Badi, Z. Molbank 2003, M0306.
- Zohdi, H. F.; Haikal, A. Molecules 2001, 6, M263.
© 2003 MDPI. All rights reserved.