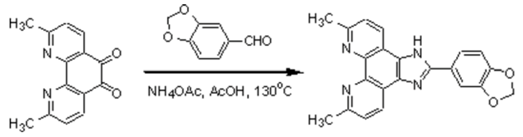
2,9-dimethyl-1,10-phenanthroline-5,6-dione was synthesized by the procedure for the preparation of 1,10-phenanthroline-5,6-dione [1]. A mixture of 3,4-methylenedioxy- benzaldehyde (0.45 g, 3 mmol), 2,9-dimethyl-1,10-phenanthroline-5,6-dione (0.714 g, 3 mmol), ammonium acetate (4.62 g, 60 mmol) and glacial acetic acid (30 cm3) was refluxed with stirring at 130 oC for 2 hours. The cooled solution was filtered, diluted with water (ca, 40 cm3) and neutralized with concentrated aqueous ammonia. The orange precipitate was collected and purified by column chromatography on alumina with ethanol-toluene (16:1 v/v) as eluent. The title compound was obtained as amorphous yellow solid (0.52 g), Yield: 47%.
Elemental analysis: Found C, 71.48; H, 4.13; N, 14.97. Calc for C22H16N4O2 C, 71.73; H, 4.38; N, 15.21.
1H NMR (500 MHz, d6-DMSO): δ14.1 (br, s, 1H), 8.90 (d, 2H), 7.84 (m, 2H), 7.81 (d, 1H), 7.79 (d, 1H), 7.18 (d, 1H), 6.09 (s, 2H), 2.50 (s, 6H).
IR (KBr, cm-1): 542(w), 629(w), 731(w), 818(s), 871(w), 931(m), 1038(s), 1102(w), 1242(s), 1351(m), 1445(s), 1472(s), 1497(s), 1585(m), 1623(w), 2776(w), 2890(w), 3067(w).
FAB-MS ([M+1]+): 369.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgement
Support for this research provided by the National Natural Science Foundation of China, the National Science Foundation of Guangdong Province and Research Fund of Royal Society of Chemistry U.K. is gratefully acknowledged.
Reference
- Yamada, M.; Tanaka, Y.; Yoshimoto, Y.; Kuroda, S.; Shimao, I. Bull. Chem. Soc. Jpn. 1992, 65, 1006.
© 2004 MDPI. All rights reserved