Benzophenone derivatives are reported to show biological activities such as cytotoxic activities against human oral squarnous carcinoma cells (HSC-2) and normal human gingival fibroblasts (HGF)1] ], antibiotic activities against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium [2] and protein kinase C inhibitor [3]. Boyd and coworkers have reported a benzophenone derivative exhibited activity in the primary anti-HIV screen [4]. Schiff bases are widely in use for synthetic purposes both by organic and inorganic chemists [5]. In addition, Schiff bases show biological activities including antibacterial [6,7], antifungal [8,9], antitumor [10,11] and herbicidal [12] activities. Schiff bases are also used as ligand for complex formation of some metal ions [13]. Metal-salen complexes are used as catalysis in epoxidation of alkenes [14], asymmetric cyclopropanation [15] and highly selective PVC membrane sensors for the sulfate ion [16]. The mentioned properties prompted us to synthesize Schiff base 3. The biological and analytical uses of Schiff base 3 are under study.
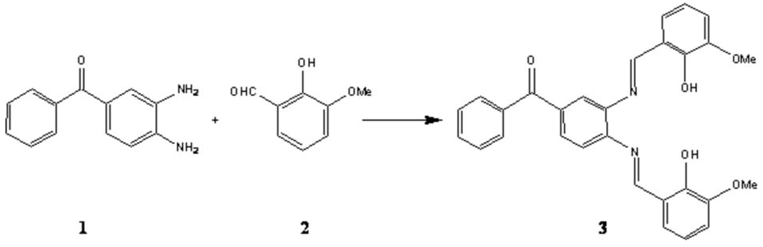
To stirred solution of o-vanillin 2 (0.85 g, 5.64 mmol) in dry dichloromethane (40.00 mL) at 0 oC were successively added 3,4-diamino benzophenone 1 (0.60 g, 2.82 mmol) and a large excces of anhydrous MgSO4 (2.00 g, 16.67 mmol). The resulting mixture was stirred for 8 hours at room temperature. The filtered solution was evaporated under reduced pressure to yield the crude Schiff base [17] which was recrystalized from ethanol 95% to give the pure Schiff base 3 as an orange solid (1.13 g, 83%).
m.p. 150-152 oC
IR (KBr) (cm-1): 1612.4 (C=N), 1678.7 (C=O), 3170.8-3656.8 (OH).
1H-NMR (CDCl3) (250 MHz) δ (ppm):6) 3.79 H, s,2 OMe), 6.70-7.96 (14H, m, aryl hydrogens), 8.53 (2H, s, 2HC=N), 12.84 (2H, br, 2 OH).
13C-NMR (CDCl3 ) (62.90 MHz) δ (ppm): 56.43 (OMe), 109.71-152.15 (aromatic carbons), 165.74 (HC=N), 197.04 (C=O).
MS (m/z, %): 480 (M+, 9.9), 344 (C6H5COC6H3N=CC6H3OHOMeN, 70.6), 239 (C6H3N=CC6H3OH OMeN, 4.6), 221 (C6H5COC6H3N=CH2N, 5.0), 180 (C6H5COC6H3, 3.2), 123 (C6H3OHOMe, 11.5), 105 (C6H5CO, 95.0), 77 (C6H5 , 100.0).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
The authors thank the Shiraz University Research Council for financial support (Grant No. 81-SC-1540-C220).
References
- Hou, A.-J.; Fukai, T.; Shimazaki, M.; Sakagami, H.; Sun, H.-D.; Nomura, T. J.Nat.Prod. 2001, 64, 65–70. [PubMed]
- Cueto, M.; Jensen, P. R.; Kauuffman, C.; Fenical, W.; Lobkovsky, E.; Clardy, J. J. Nat. Prod. 2001, 64, 1444–1446. [PubMed]
- Storm, J. P.; Andersson, C.-M. Org. Lett. 1999, 1(9), 1451–1453. [PubMed]
- Boyd, M. R.; Fuller, R. W.; Westergaard, C. K.; Collines, J. W.; Cardllina, J.H., II. J. Nat. Prod. 1999, 62, 67–69.
- Arora, K.; Gupta, A.; Agarwal, D.D. Asian J. Chem. 2002, 14, 1611–1615.
- Saravanan, J.; Mohan, S. Asian J. Chem. 2003, 15, 67–70.
- Pandeya, S. N.; Sriram, D.; Nath, G.; De Clercq, E. IL Farmaco 1999, 54, 624–628. [PubMed]
- Berad, B.N.; Deshmukh, M.R.S.; Bhaskar, C.S. Asian J. Chem. 2002, 14, 1241–1245.
- Krishnaiah, Y.S.R.; Lakashmi, M.; Satyanarayana, V.; Bhashkar, P. Asian J. Chem. 2002, 14, 1246–1250.
- Hodnett, E. M.; Dunn, W. J. J. Med. Chem. 1970, 13, 768–770. [PubMed]
- Nofal, Z. M.; El-Zahar, M. I.; Abd El-Karim, S. S. Molecules 2000, 5, 99–113.
- Samadhiya, S.; Halve, A. Orient. J. Chem. 2001, 17(1), 119–122.
- Tai, X.; Yin, X.; Chen, Q.; Tan, M. Molecules 2003, 8, 439–443.
- Abashkin, Y. G.; Burt, S. K. Org. Lett. 2004, 6(1), 59–62. [PubMed]
- Nguyen, S. B. T.; Miller, J. A.; Hennessy, E.J.; Marshall, W. J.; Scialdone, M.A. J. Org. Chem. 2003, 68, 7884–7886.
- Shamsipur, M.; Yousefi, M.; Hosseini, M.; Ganjali, M. R.; Shargi, H.; Naeimi, H. Anal. Chem. 2001, 73, 2869–2874. [PubMed]
- Matsui, S.; Hashimoto, Y.; Saigo, K. Synthesis 1998, 1161–1166.
- Sample Availability : Available from MDPI.
© 2004 MDPI. All rights reserved