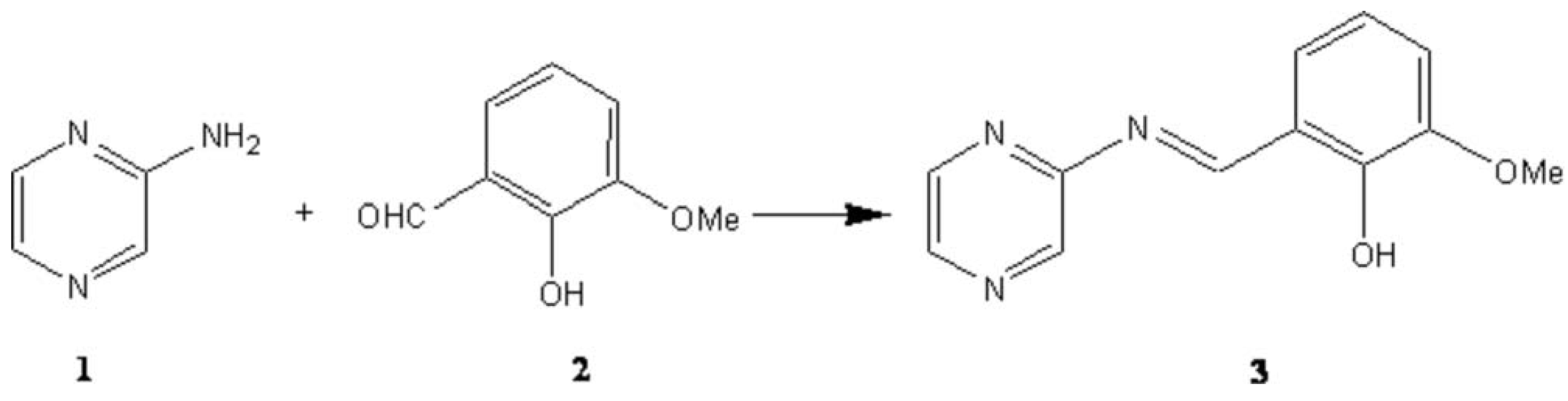
Synthesis of 2-methoxy-6-(pyrazin-2-ylimino methyl) phenol and its antibacterial activitity
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References
- Buchbauer, G.; Klein, C.Th.; Wailzer, B.; Wolschann, P. J. Agric. Food Chem. 2000, 48, 4273–4278. [CrossRef]
- Chi-Kuen, Shu. J. Agric. Food Chem. 1999, 47, 4332–4335. [PubMed]
- Dolezal, M.; Miletin, M.; Kunes, J.; Kralova, K. Molecules 2002, 7, 363–373.
- Gezginci, M. H.; Martin, A. R.; Franzblau, S. G. J. Med. Chem 2001, 44, 1560–1563. [CrossRef]
- Jeanjot, P.; Bruyneel, F.; Arrault, A.; Gharbi, S.; Cavalier, J.-F.; Abels, A.; Marchand, C.; Touillaux, R.; Rees, J.-F.; Marchand-Brynaert, J. Synthesis 2003, (4), 513–522.
- Cavalier, J.-F.; Burton, M.; De Tollenaere, C.; Dussart, F.; Marchand, C.; Rees, J.-F.; Marchand- Brynaert, J. Synthesis 2001, (5), 768–772.
- Tai, X.; Yin, X.; Chen, Q.; Tan, M. Molecules 2003, 8, 439–443. [CrossRef]
- Kabeer, A. S.; Baseer, M. A.; Mote, N. A. Asian J. Chem. 2001, 13(2), 496–500.
- More, P. G.; Bhalvankar, R. B.; Pattar, S. C. J. Indian Chem. Soc. 2001, 78(9), 474–475.
- Pandeya, S. N.; Sriram, D.; Nath, G.; De Clercq, E. IL Farmaco 1999, 54, 624–628. [PubMed]
- Singh, W. M.; Dash, B. C. Pesticides 1988, 22(11), 33–37.
- Hodnett, E. M.; Dunn, W. J. J. Med. Chem. 1970, 13, 768–770. [CrossRef] [PubMed]
- Pathak, P.; Jolly, V. S.; Sharma, K. P. Orient. J. Chem. 2000, 16(1), 161–162.
- Samadhiya, S.; Halve, A. Orient. J. Chem. 2001, 17(1), 119–122.
- Aydogan, F.; Öcal, N.; Turgut, Z.; Yolacan, C. Bull. Korean Chem. Soc. 2001, 22, 476–480.
- Halve, A.; Goyal, A. Orient. J. Chem. 1996, 12(1), 87–88.
- Sample Availability : Available from MDPI.
© 2004 MDPI. All rights reserved
Share and Cite
Jarrahpour, A.A.; Motamedifar, M.; Hadi, N.; Zarei, M. Synthesis of 2-methoxy-6-(pyrazin-2-ylimino methyl) phenol and its antibacterial activitity. Molbank 2004, 2004, M373. https://doi.org/10.3390/M373
Jarrahpour AA, Motamedifar M, Hadi N, Zarei M. Synthesis of 2-methoxy-6-(pyrazin-2-ylimino methyl) phenol and its antibacterial activitity. Molbank. 2004; 2004(1):M373. https://doi.org/10.3390/M373
Chicago/Turabian StyleJarrahpour, A. A., M. Motamedifar, N. Hadi, and M. Zarei. 2004. "Synthesis of 2-methoxy-6-(pyrazin-2-ylimino methyl) phenol and its antibacterial activitity" Molbank 2004, no. 1: M373. https://doi.org/10.3390/M373
APA StyleJarrahpour, A. A., Motamedifar, M., Hadi, N., & Zarei, M. (2004). Synthesis of 2-methoxy-6-(pyrazin-2-ylimino methyl) phenol and its antibacterial activitity. Molbank, 2004(1), M373. https://doi.org/10.3390/M373



