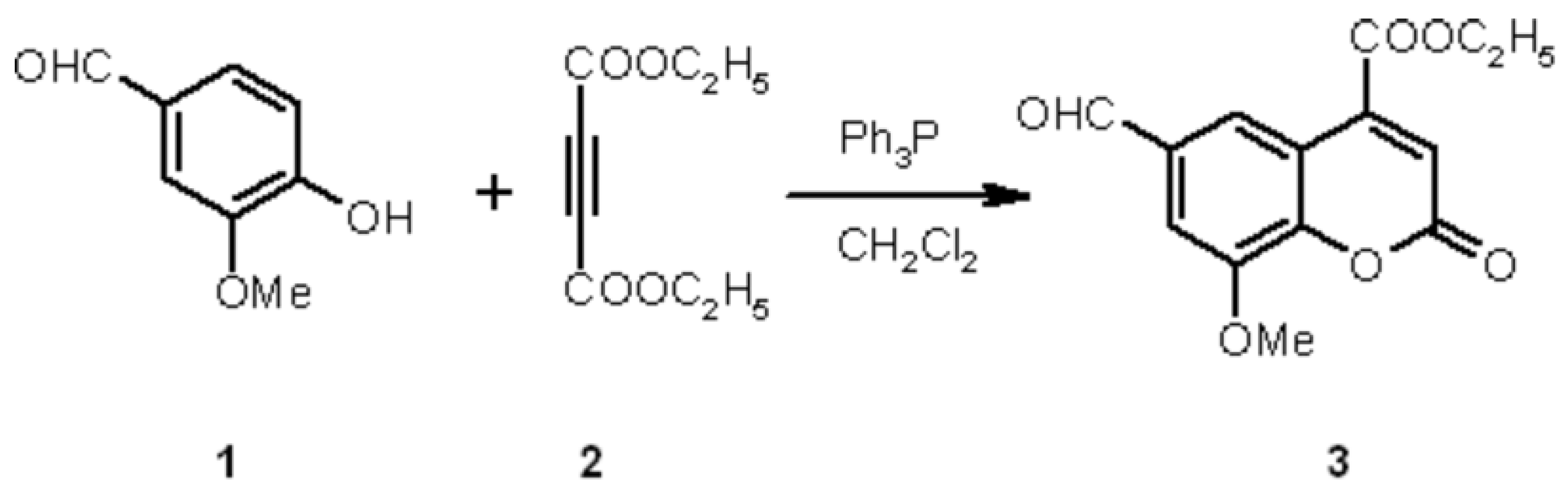
A mixture of vanillin 1 ( 0.5g, 3.3mmol) and triphenylphosphine ( 0.86g, 3.3mmol) was dissolved in CH2Cl2. The reaction mixture was cooled in ice bath to -5 °C. Dimethylacetylenedicarboxylate 2 ( 0.56g, 3.3mmol) in CH2Cl2 was added dropwise over a period of 10 min under stirring. The reaction mixture was then refluxed for 4-5 hours, the solvent was removed under reduced pressure and the solid mass was purified by recrystallization from ethanol to give the desired product as yellow crystals ( 0.45g, 50%).
M.p. 118 °C ( recrystallized from EtOH, uncorrected).
UV lmax (nm; EtOH)/e (dm3·mol−1·cm−1) 335/15875
IR (cm−1, KBr) 2980, 2863 (C-H, aldehydic), 1725 (C=O), 1600 (C=C).
1H-NMR (400 MHz; CDCl3; Me4Si) dH 9.99 (s, 1H, CHO), 8.44 ( d, 1H, J = 1.3 Hz, H-3), 7.62 ( d, 1H, J= 1.3 Hz, H-5), 7.08 (s, 1H, H-7), 4.5 (q, 2H, J = 7.0 Hz, CH2O), 4.02 (s, 3H, CH3O), 1.47 (t, 3H, J = 7.0 Hz, CH3).
13C-NMR (100 MHz; CDCl3; Me4Si) d 13.85, 56.22, 62.67, 110.33 , 116.29, 120.26, 123.33, 128.19, 132.44, 141.67, 147.83, 158.31, 163.02, 190.31.
Anal.Calc. for C14H12O6 ( 276.241): C 60.87, H 4.38; found : C 60.61, H 4.23.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Issa, Y.; Rahim, H-S.; Afsaneh, Z. Tetrahedron Letters 1998, 39(16), 2394–2392.
- Johnson, J. R. Org. Reaction 1942, Vol. 1, 210–265.
© 2004 MDPI. All rights reserved.