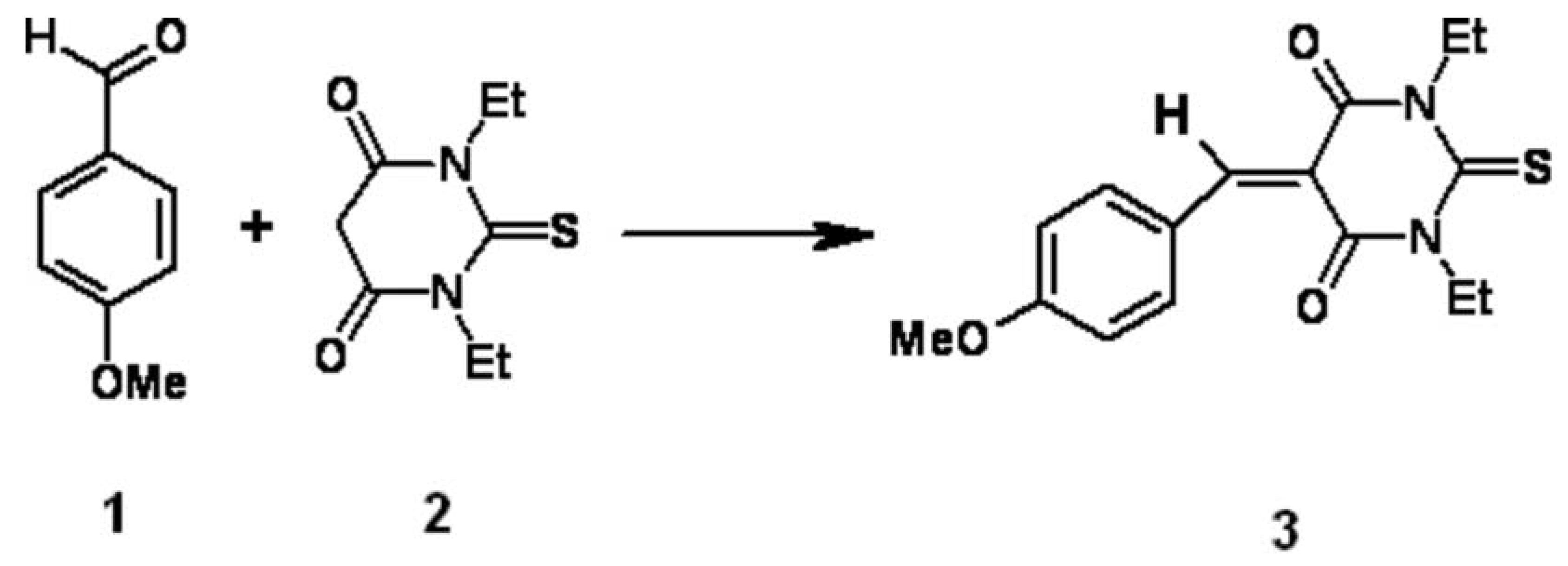
1,3-Diethyl-5-(4-methoxybenzylidene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione 3 was prepared by Knoevenagel condensation of 4-methoxybenzaldehyde 1 and N,N-diethylthiobarbituric acid 2 in ethanol using piperidine as a base [1,2].
N,N-Diethylthiobarbituric acid 2 ( 7.5 g, 0.0375 mol) and 4-methoxybenzaldehyde 1 (5.1 g, 0.0375 mmol ) in ethanol (75 mL) were heated under reflux for five minutes. Piperidine (1.0 mL) was added in one portion and the reflux was continued for five hours. The reaction mixture was cooled to room temperature and the solid formed was filtered, washed with cold ethanol (2x 40 mL) and dried. 1,3-Diethyl-5-(4-methoxybenzylidene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione 3 was recrystallized from ethanol as yellow crystals (10.86g, 91%).
M.p. 147 °C ( uncorrected.).
UV lmax (nm; CHCl3)/e (dm3.mol-1.cm-1) 414/25000 and 258/ 5848.
IR (KBr) cm-1 2978, 1661 (C=O), 1602 (C=C), 1433.5, 1387 (CS).
Theoretical (cm-1; AM1): 1650.6; 1462.1; 1388.8
1H-NMR (400 MHz; CDCL3; Me4Si) dH 8.52 (1H, s, -CH=C), 8.37 (2H, d, J = 8.94 Hz, aromatic), 7.00(2H, d, J = 7.24 Hz, J = 8.94 Hz, aromatic), 4.58 ( 4H, t, 2xCH2), 3,90 (3H, s, MeO), 1.32 ( 6H, q, CH3).
13C-NMR (100 MHz; CDCL3; Me4Si) dC 174.9 (C=S), 163.8, 163.4 (C=O), 157.7, 137.7 (CH oliefinc), 155.6, 132.4, 128.2, 126.5 (C), 114.5, 56.03 (MeO), 44.6, 44.3 (CH2), 13.5, 13.2 (CH3).
Anal.Calc. for C16H18N2O3S ( 318.392): C 60.36, H 5.70 , N 10.07; found : C 60.15, H 5.5.79, N 10.20.
Theoretical calculations were performed with the GAUSSIAN 98 package [3] using the AM1 semi-empirical model [4]. From the above (AM1 values) we see that the theoretical and experimental values are in good agreement. Using a rigid-rotor/harmonic-oscillator (RRHO) approximation in agreement with NIST standards we compute at 298.15 K, S (entropy in J mol-1 K-1) as 674.66, Cp as 329.70 (heat capacity at constant pressure in kJ mol-1 K-1) and H° - H°298.15 as 58.50 (enthalpy content, in kJ mol-1). The equations most useful for indexing these species with the NIST are:
| Fitted Thermodynamic Equation (T/1000=t) | T1 | T2 | T3 | |
| S | -7.63127*ln(t) + 1284.11508*t -578.09841*t2/2 + 33.99402*t3/3 + 0.57583/(2*t2) +311.06252 | 425.38 | 674.66 | 1317.25 |
| Cp | 329.41584+ 1243.04945*t -253.18884*t2 -1.8789*t3 -0.25805*t-2 | 157.48 | 329.70 | 738.27 |
| DH | 148.67084*t + 5284.44948*t2/2 -9023.3457*t3/3 + 4547.86803*t4/4 + 16.23129 | 10.27 | 58.50 | 460.39 |
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Jones, G. Org. React. 1967, 15, 204.
- Tanaka, K.; Chen, X.; Yoneda, F. Tetrahedron 1988, 44, 3241.
- Frisch, M.J.; et al. Gaussian 98,Revision A.6. Gaussian, Inc.: Pittsburgh PA, 1998. [Google Scholar]
- Foresman, J.B. Æ Frisch, Exploring Chemistry with Electronic Structure Methods, 2nd editionGaussian, INC: Pittsburgh, PA, 1996. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69. 2001.
© 2004 MDPI. All rights reserved.