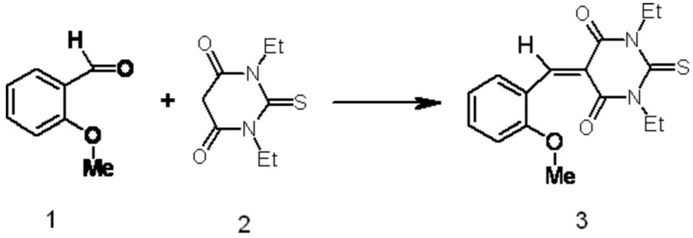
1,3-Diethyl-5-(2-methoxybenzylidene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Jones, G. Org. React. 1967, 15, 204–599.
- Tanaka, K.; Chen, X.; Yoneda, F. Tetrahedron 1988, 44, 3241–3249.
© 2004 MDPI. All rights reserved.
Share and Cite
Asiri, A.M.; Alamry, K.A.; Jalbout, A.F.; Zhang, S. 1,3-Diethyl-5-(2-methoxybenzylidene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione. Molbank 2004, 2004, M359. https://doi.org/10.3390/M359
Asiri AM, Alamry KA, Jalbout AF, Zhang S. 1,3-Diethyl-5-(2-methoxybenzylidene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione. Molbank. 2004; 2004(1):M359. https://doi.org/10.3390/M359
Chicago/Turabian StyleAsiri, Abdullah Mohamed, Khaled Ahmed Alamry, Abraham F. Jalbout, and Suhong Zhang. 2004. "1,3-Diethyl-5-(2-methoxybenzylidene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione" Molbank 2004, no. 1: M359. https://doi.org/10.3390/M359
APA StyleAsiri, A. M., Alamry, K. A., Jalbout, A. F., & Zhang, S. (2004). 1,3-Diethyl-5-(2-methoxybenzylidene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione. Molbank, 2004(1), M359. https://doi.org/10.3390/M359



