Recently we have reported [
1] six new cell cycle inhibitors belonging to plant polyphenolics from
Rubus aleaefolius Poir. (family
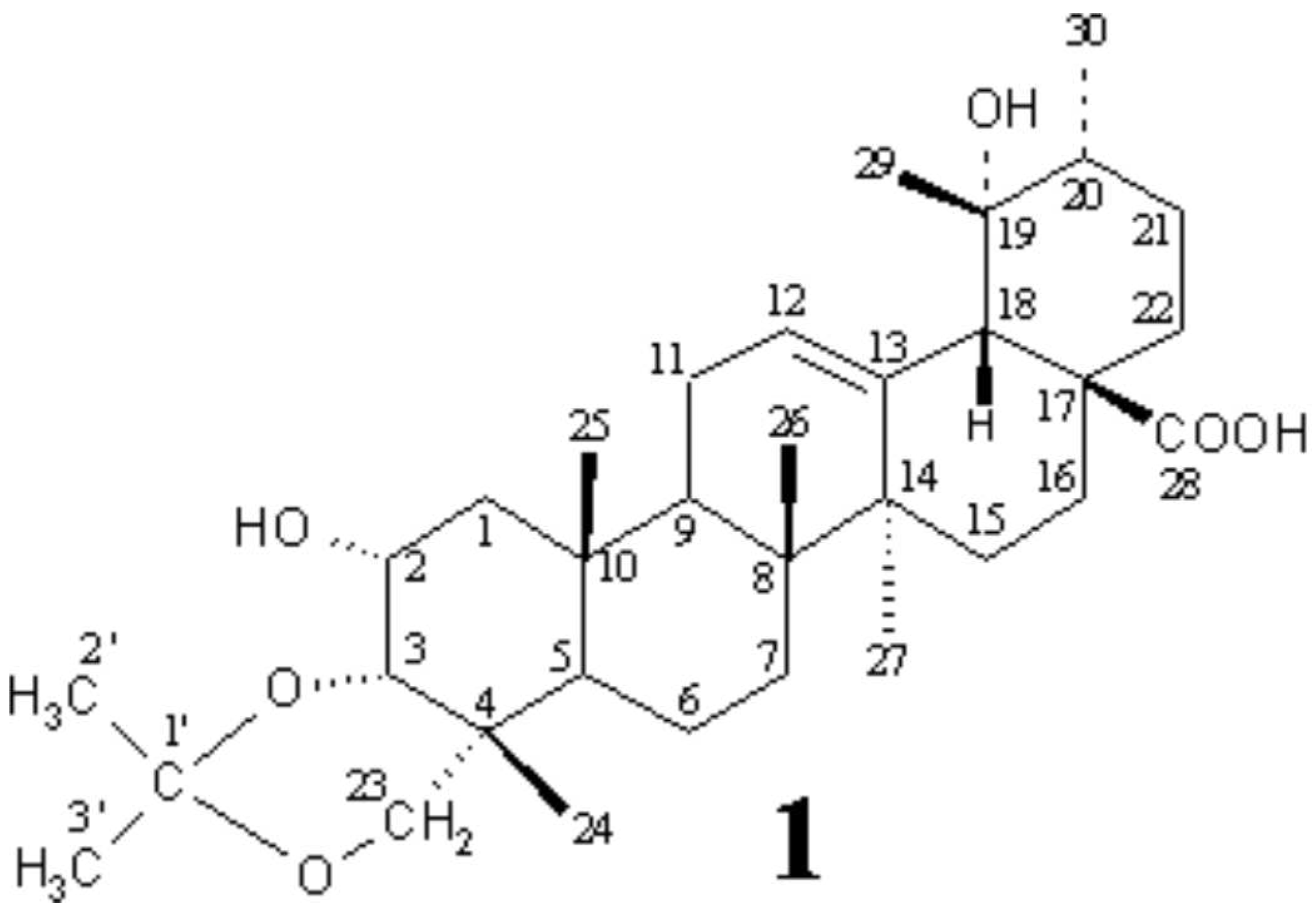
Rosacae), the source plant of a traditional Chinese medicine Cuye Xuangouzi, which is used also as a folk medicine to cure certain cancers in partial area of China. In a continuation of that work, this communication describes a new pentacyclic triterpene, 3α,23-
O-isopropylidenyl-2α,19α-dihydroxy-urs-12-en-28-oic acid (1), obtained from
Rubus aleaefolius Poir., which inhibits mammalian cell cycle at G0/G1 phase.
The roots (3 kg) of
R. aleaefolius were extracted with 60% aqueous alcohol to give an alcoholic extract (435 g) possessing potential inhibitory activity on the cell cycle of tsFT210 cells [
1]. The alcoholic extract was thus suspended in water and then extracted successively with chloroform and ethyl acetate to obtain an active ethyl acetate extract (78 g). This extract was further separated by repeated solvent-extraction, silica gel column chromatography, preparative HPLC and recrystallization procedure in a bioassay-guided manner to obtain pure
1 (26.8 mg).
Compound 1, white crystals (from MeOH), mp 196-198°C, [α]25D +31.7° (c 0.85, MeOH), gave positive Libermann-Burchard reaction, showing typical color for triterpenoids, and its molecular formula, C33H52O6 (molecular weight 544), was determined by negative HR-SIMS-MS measurement. The IR spectrum of 1 indicated the presence of OH (3449 cm-1), COOH (3400-2400 br and 1702 cm-1), C=C (1638 cm-1), and C-O (1040 cm-1) groups in 1.
The
1H and
13C NMR spectra of
1 in CDCl
3, analyzed by DEPT and PFG 2D NMR (
1H-
1H COSY, HMQC, HMBC and NOESY) techniques, resembled those of known 2α,3α,19α,23-tetrahydroxy-urs-12-en-28-oic acid [
2] (
2) except for additional signals due to an extra
O-isopropylidenyl group (δ
H1.42 s, 2'-H
3, δ
C 19.22 q, C-2'; δ
H 1.40 s, 3'-H3, δ
C 29.38 q, C-3'; and δ
C 98.67 s, C-1') [
3] in
1, indicating that
1 is an isopropylidenyl derivative of
2. Location of the
O-isopropylidenyl group at C-3 and C-23 positions could be determined on the basis of HMBC correlations between 3-H and C-1' and between 23-H and C-1'. The
cis-relation between β-axial 2-H and β-equatorial 3-H was evidenced by the chemical shift and J values of 2-H (δ 3.88 ddd, J=12.1, 4.1, 2.8 Hz) and 3-H (δ 3.77 d, J=2.8 Hz) [
4]. The chemical shift and J values of α-axial 16-H (δ 2.53 td, J=13.4, 4.4 Hz) established both the 19α-OH stereochemistry and the
cis-stereochemistry of the ring-D/E junction [
5]. And eventually the structure of
1 was established as 3α,23-
O-isopropylidenyl-2α,19α-dihydroxy-urs-12-en-28-oic acid.
Compound 1 inhibited the cell cycle progression of asynchronously cultured tsFT210 cells at the G0/G1 phase with the MIC value of 183.8 μmol/L.
UV λmax nm (log ε) in MeOH: 206 (3.71).
IR νmax cm-1 (KBr): 3449 (OH), 3400-2400 br (COOH), 2987, 2973, 2936, 2877 (CH3 & CH2), 1702 (COOH), 1638 (C=C), 1459, 1380 (CH3 & CH2), 1200, 1150, 1040 (C-O).
Positive ESI-MS m/z: 567 [M+Na]+; negative ESI-MS m/z: 543 [M-H]-; negative HR-SIMS-MS m/z: 543.3686 (calcd for C33H51O6 [M-H]- 543.3690).
1H-NMR (600 MHz, CDCl3): δ 1.28 (t, J=12.1 Hz, 1-Hax.), 1.71 (dd, J=ca.12.1, 4.1 Hz, 1-Heq.), 3.88 (ddd, J=12.1, 4.1, 2.8 Hz, 2-H), 3.77 (d, J=2.8 Hz, 3-H), 1.78 (br d, J=12.4 Hz, 5-H), 1.27 and 1.42 (both m, 6-H2), 1.30 (m, 7-Heq.), 1.63 (td, J=12.6, 4.1 Hz, 7-Hax.), 1.81 (dd, J=ca.11.1, 6.6 Hz, 9-H), 2.06 (ddd, J=18.7, 6.6, 3.3 Hz, 11-Heq.), 1.99 (ddd, J=18.7, 11.1, 3.7 Hz, 11-Hax.), 5.36 (dd, J=3.7, 3.3 Hz, 12-H), 1.04 (br d, J=12.7 Hz, 15-Heq.), 1.78 (ddd, J=13.4, 12.7, 4.1 Hz, 15-Hax.), 2.53 (td, J=13.4, 4.4 Hz, 16-Hax.), 1.58 (dm, J=13.4 Hz, 16-Heq.), 2.536 (s, 18-H), 1.39 (m, 20-H), 1.28 and 1.71 (both m, 21-H2), 1.66 and 1.80 (both m, 22-H2), 3.30 and 3.66 (both d, both J=12.1 Hz, 23-H2), 0.71 (3H, s, 24-H3), 0.97 (3H, s, 25-H3), 0.72 (3H, s, 26-H3), 1.29 (3H, s, 27-H3), 1.21 (3H, s, 29-H3), 0.95 (3H, d, J=6.6 Hz, 30-H3), 1.42 (3H, s, 2'-H3), 1.40 (3H, s, 3'-H3). The above signal assignments were based on the results of PFG 2D NMR (1H-1H COSY, HMQC, HMBC and NOESY) experiments.
13C-NMR (150 MHz, CDCl3): δ 42.36 t (C-1), 65.33 d (C-2), 76.01 d (C-3), 36.12 s (C-4), 42.07 d (C-5), 17.76 t (C-6), 32.47 t (C-7), 40.27 s (C-8), 47.12 d (C-9), 38.12 s (C-10), 23.72 t (C-11), 129.34 d (C-12), 138.05 s (C-13), 41.15 s (C-14), 28.24 t (C-15), 25.41 t (C-16), 47.81 s (C-17), 52.92 d (C-18), 73.17 s (C-19), 41.24 d (C-20), 26.05 t (C-21), 37.56 t (C-22), 68.20 t (C-23), 16.92 q (C-24), 17.02 q (C-25), 17.24 q (C-26), 24.66 q (C-27), 184.13 s (C-28), 27.49 q (C-29), 16.25 q (C-30), 98.68 s (C-1'), 19.22 q (C-2'), 29.38 q (C-3'). The above signal assignments were based on the results of DEPT and PFG 2D NMR (1H-1H COSY, HMQC, HMBC and NOESY) experiments.