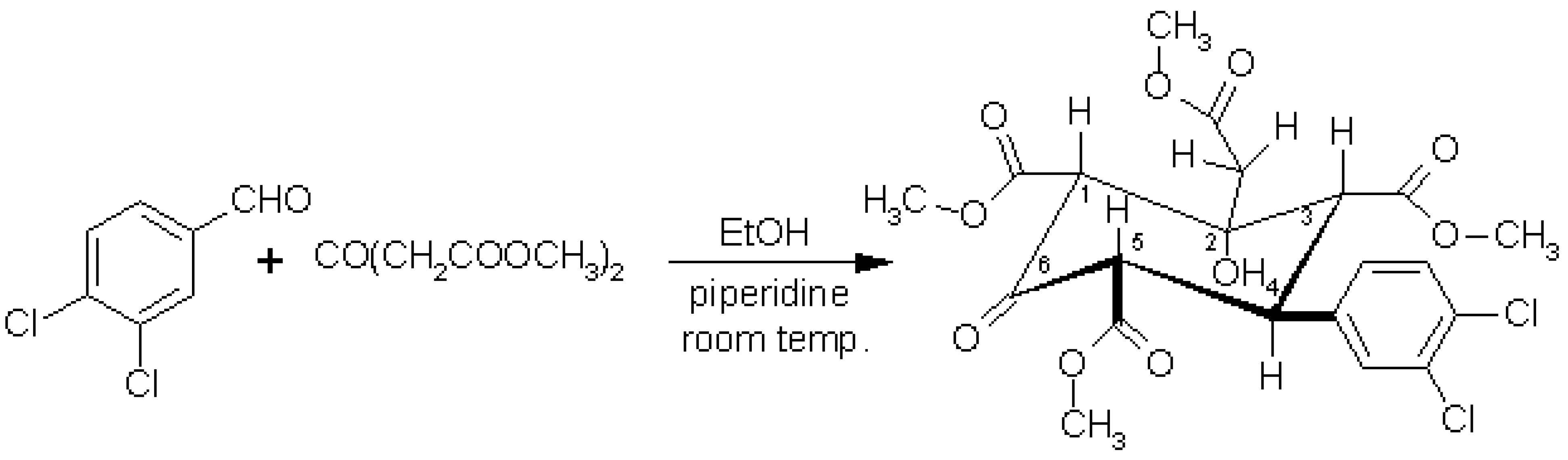
Trimethyl 4-(3,4-Dichlorophenyl)-2-hydroxy-2-(2-methoxy-2-oxoethyl)-6-oxo-1,3,5-cyclohexanetricarboxylate
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Haensel, W.; Haller, R. Arch. Pharm. (Weinheim Ger.) 1970, 303, 334–338.
- Sample Availability: Available from the authors and from MDPI.
© 2003 MDPI. All rights reserved.
Share and Cite
Stoyanov, E.V. Trimethyl 4-(3,4-Dichlorophenyl)-2-hydroxy-2-(2-methoxy-2-oxoethyl)-6-oxo-1,3,5-cyclohexanetricarboxylate. Molbank 2003, 2003, M325. https://doi.org/10.3390/M325
Stoyanov EV. Trimethyl 4-(3,4-Dichlorophenyl)-2-hydroxy-2-(2-methoxy-2-oxoethyl)-6-oxo-1,3,5-cyclohexanetricarboxylate. Molbank. 2003; 2003(2):M325. https://doi.org/10.3390/M325
Chicago/Turabian StyleStoyanov, Edmont V. 2003. "Trimethyl 4-(3,4-Dichlorophenyl)-2-hydroxy-2-(2-methoxy-2-oxoethyl)-6-oxo-1,3,5-cyclohexanetricarboxylate" Molbank 2003, no. 2: M325. https://doi.org/10.3390/M325
APA StyleStoyanov, E. V. (2003). Trimethyl 4-(3,4-Dichlorophenyl)-2-hydroxy-2-(2-methoxy-2-oxoethyl)-6-oxo-1,3,5-cyclohexanetricarboxylate. Molbank, 2003(2), M325. https://doi.org/10.3390/M325



