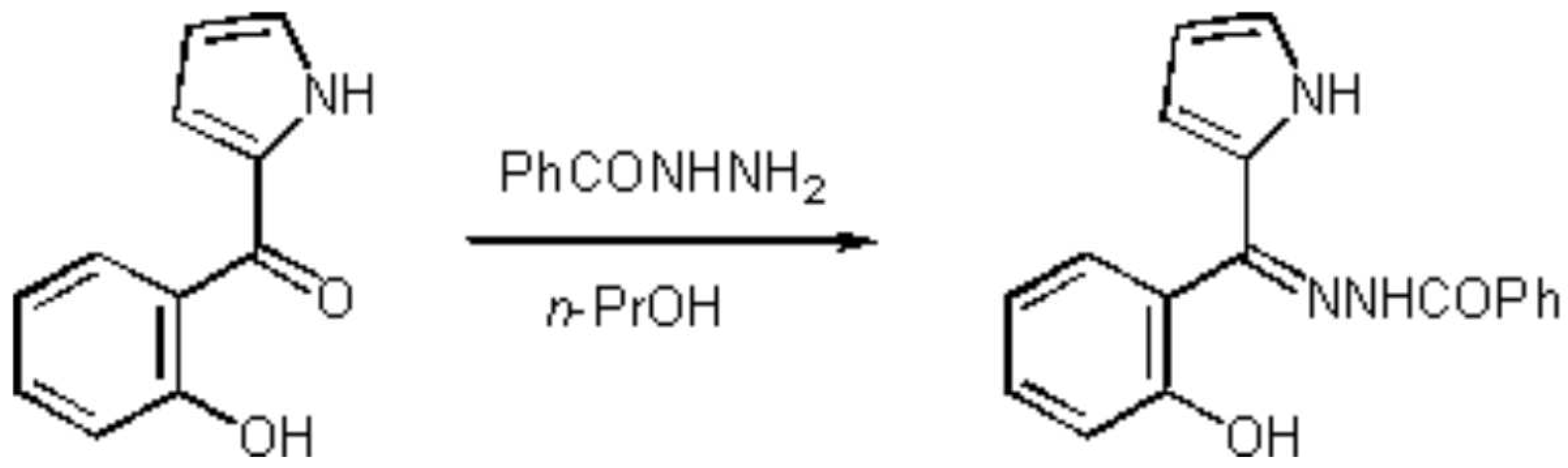
2-(2´-Hydroxybenzoyl)pyrrole N-Benzoylhydrazone
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Sample availability: available from the authors and MDPI.
© 2003 MDPI. All rights reserved.
Share and Cite
Kotali, A. 2-(2´-Hydroxybenzoyl)pyrrole N-Benzoylhydrazone. Molbank 2003, 2003, M351. https://doi.org/10.3390/M351
Kotali A. 2-(2´-Hydroxybenzoyl)pyrrole N-Benzoylhydrazone. Molbank. 2003; 2003(4):M351. https://doi.org/10.3390/M351
Chicago/Turabian StyleKotali, Antigoni. 2003. "2-(2´-Hydroxybenzoyl)pyrrole N-Benzoylhydrazone" Molbank 2003, no. 4: M351. https://doi.org/10.3390/M351
APA StyleKotali, A. (2003). 2-(2´-Hydroxybenzoyl)pyrrole N-Benzoylhydrazone. Molbank, 2003(4), M351. https://doi.org/10.3390/M351



