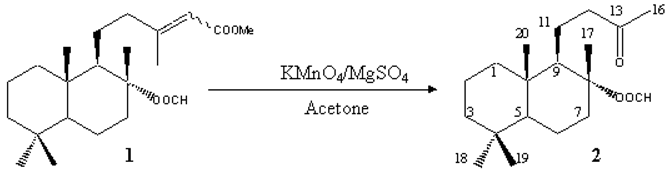
To a stirred solution of a mixture of E/Z isomers 1 (81 mg, 0.22 mmol, 45:55 ratio) in acetone (5 mL) was added a mixture of KMnO4 (122 mg, 0.77 mmol) and anhydrous MgSO4 (120 mg) at 10 °C [1]. After stirring for 10 min reaction was allowed to warm to room temperature. Then another portion of KMnO4 (25 mg, 0.16 mmol) and anhydrous MgSO4 (25 mg) was added. After 20 min the crude was filtered over Celite and the clean solution evaporated under reduced pressure to yield a residue which was solved in Et2O (25 mL). This solution was washed with brine (3×10 mL), dried over anhydrous Na2SO4 and the solvent evaporated under reduced pressure to yield a residue (61 mg) which was purified by flash chromatography on silica gel, using a 2:3 hexane/Et2O mixture as eluent, to give the title compound 2 (50 mg, 0.16 mmol, 73%).
Mp: 66.0-67.4 °C (white crystals, from hexane).
[a]D = -22.3° (c 1.01 cg·mL-1, CHCl3).
IR (KBr, n, cm-1): 1722, 1198, 1165 (OOCH), 1696 (CO).
1H NMR (300 MHz, CDCl3, d, ppm): 0.79 (3H, s, Meb-4), 0.86 (3H, s, Me-10), 0.88 (3H, s, Mea-4), 1.52 (3H, s, Me-8), 2.13 (3H, s, Me-13), 0.93-1.84 (13H, m, H-1,2,3,5,6,7a,9,11), 2.43-2.69 (3H, m, Hb-7, H-12), 8.02 (1H, s, OOCH).
13C NMR (75 MHz, CDCl3, d, ppm): 39.48 (C-1), 18.17 (C-2), 41.67 (C-3), 33.04 (C-4), 55.50 (C-5), 19.87 (C-6), 39.48 (C-7), 88.99 (C-8), 57.95 (C-9), 39.48 (C-10), 19.26 (C-11), 46.37 (C-12), 208.98 (C-13), 29.78 (C-16), 21.03 (C-17), 33.21 (C-18), 21.32 (C-19), 15.42 (C-20), 160.28 (OOCH).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
We wish to thank the Junta de Andalucía for financial support and the Ministerio de Educación, Cultura y Deporte for a Fellowship to J. M. Castro.
References and Notes
- Leite, M. A. F.; Sarragiotto, M. H.; Imamura, P. M.; Marsaioli, A. J. Absolute Configuration of Drim-9(11)-en-8-ol from Aspergillus oryzae. J. Org. Chem. 1986, 51, 5409–5410. [Google Scholar] [CrossRef]
- Sample availability: Available from the authors and from MDPI
© 2003 MDPI. All rights reserved.